Experimental Investigation of the Glass Transition Temperature in Amorphous Selenium under High Pressures
-
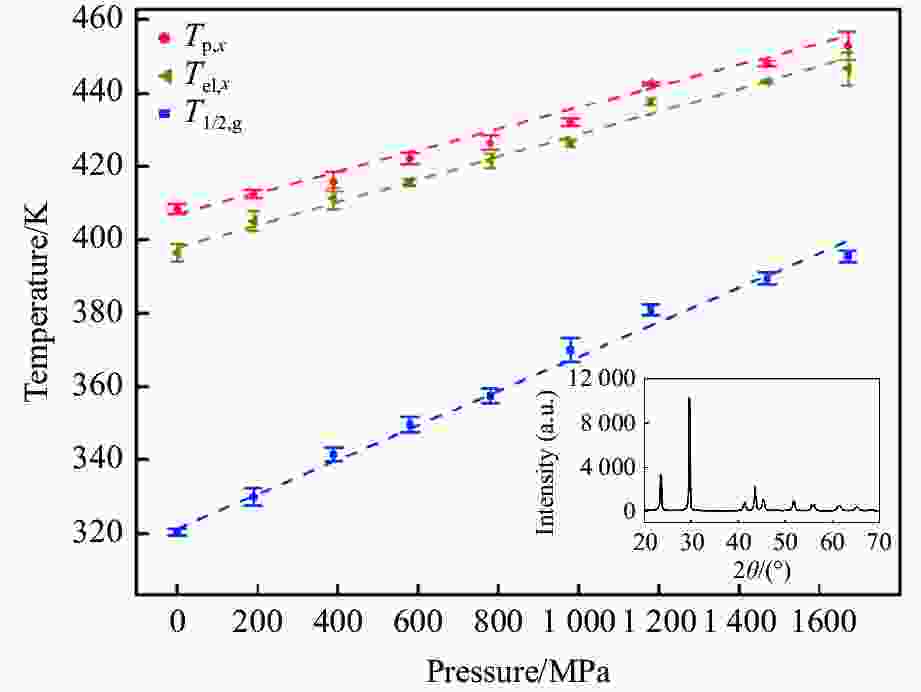
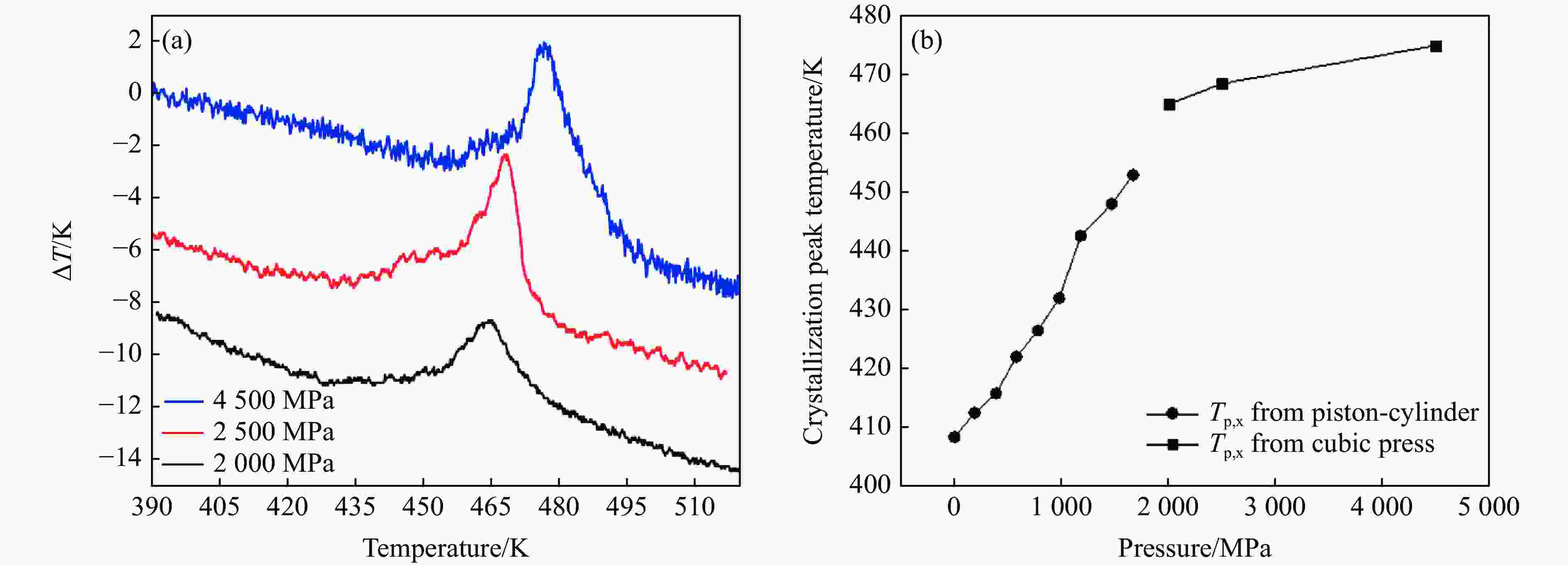
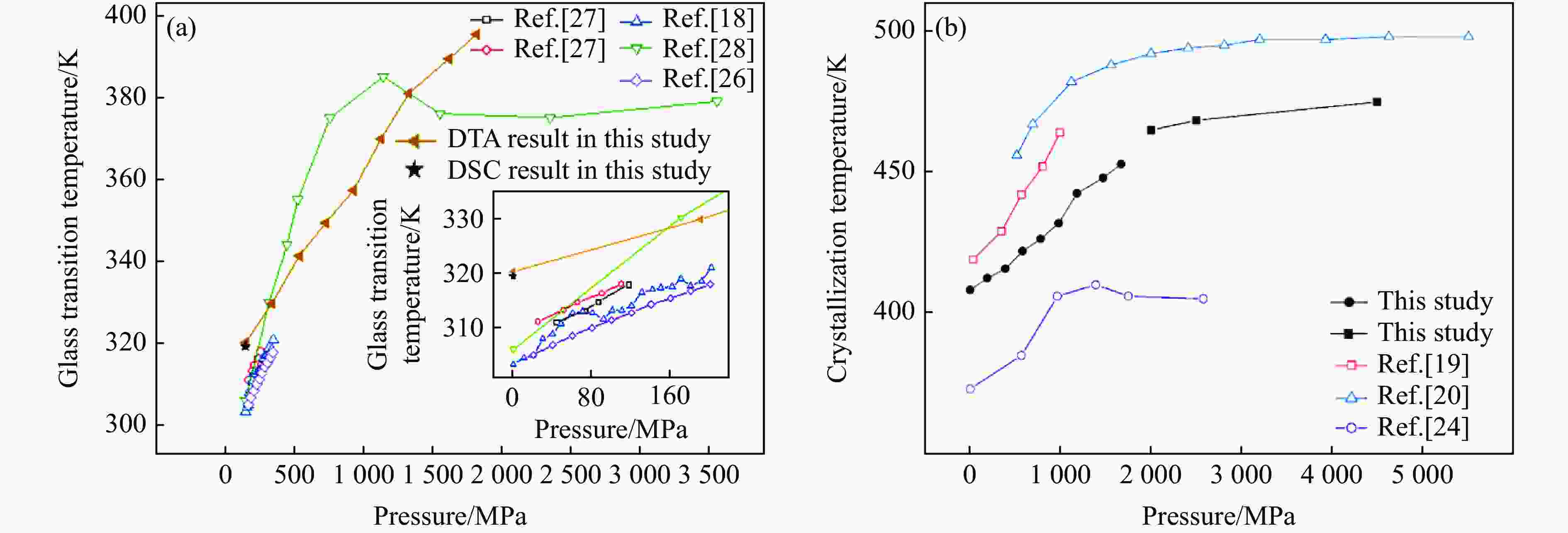
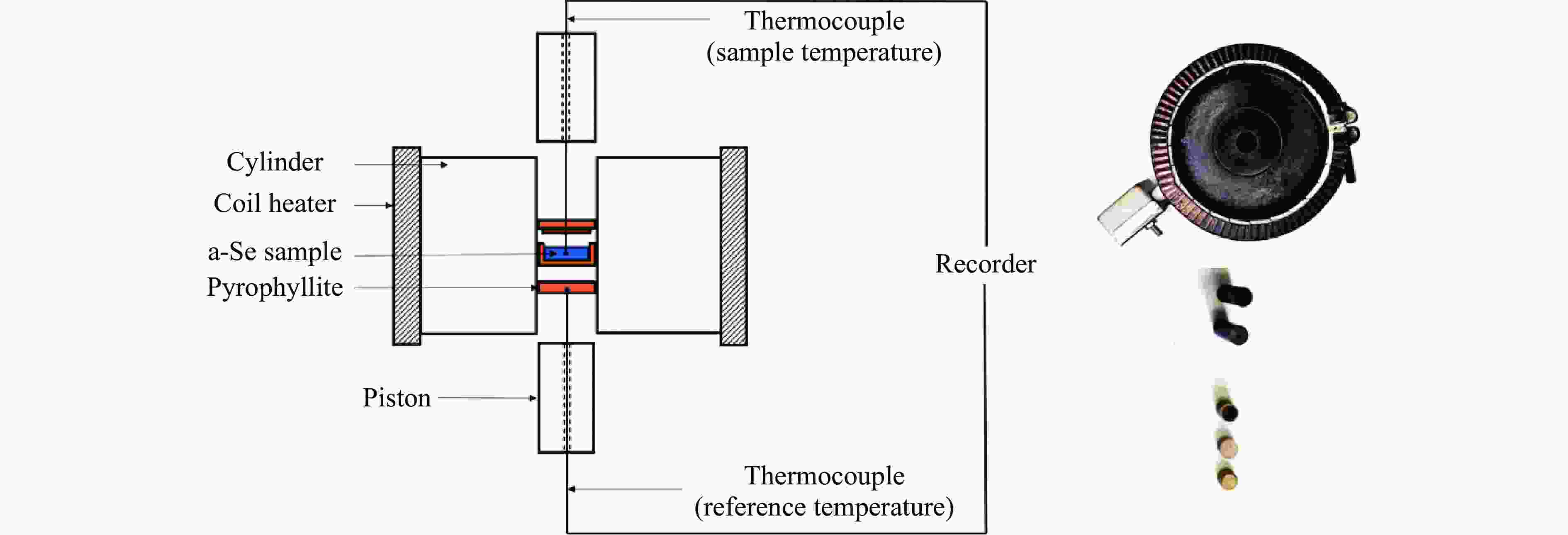
摘要: 针对熔体急冷法制备的非晶硒样品,开展了压力对非晶硒玻璃化转变温度和过冷液相区影响的实验研究。在活塞圆筒高压模具上开展差热分析,测得了0.1~1700 MPa压力范围内非晶硒的玻璃化转变温度
$ {T}_{\text{g}} $ 和晶化温度${T}_{x}$ ,拟合出玻璃化转变中点温度$ {T}_{1/2,\text{g}} $ 与外推起始晶化温度Tel,x随压力p的变化关系:${T}_{1/2,\text{g}}\left(p\right)=$ $322+0.046\,2p$ ,${T}_{\mathrm{e}\mathrm{l},x}\left(p\right)=398+0.030\,2p$ ,其中,$ {T}_{1/2,\text{g}} $ 和Tel,x的单位均为K,p的单位为MPa。$ {T}_{1/2,\text{g}} $ 和Tel,x均随压力的增加而升高。由于Tel,x(p)的斜率小于$ {T}_{1/2,\text{g}} $ (p)的斜率,导致过冷液相区的温度范围随着压力的增加而变窄。在六面顶压机上开展差热分析,测得了2000~4500 MPa压力范围内非晶硒的晶化温度。结合活塞圆筒实验结果,发现了非晶硒的晶化温度随压力的变化规律:在0.1~1700 MPa范围内,晶化温度随压力的增加而升高;在2000 MPa以上,晶化温度的上升速率随压力的增加明显降低。当压力引起非晶硒的微观结构变化时,Tg(p)与Tx(p)曲线的斜率变化发生在相近的压力下,结合实验结果—Tx(p)的斜率变化出现在2 GPa左右,因此,推测Tg(p)的斜率变化可能出现在2 GPa左右。大腔体高压装置实验获得的转变点压力与以往报道的金刚石压砧实验结果不一致,可能与这两类实验中玻璃化转变温度、晶化温度的测量方法不同及压力测量误差有关。Abstract: The effect of pressure on the glass transition temperature and the supercooled liquid region of amorphous selenium (a-Se), which was prepared through melting quenching, was investigated. The glass transition temperature$ {T}_{\text{g}} $ and crystallization temperature Tx were determined through the differential thermal analysis (DTA) during isobaric heating. The experimental results from piston-cylinder apparatus showed that both$ {T}_{\text{g}} $ and Tx increase with the increasing pressure in the pressure range of 0.1-1700 MPa. The glass transition middle temperatures$ {T}_{1/2,\text{g}} $ and extrapolated crystallization onset temperatures Tel,x were linearly fitted to pressure. The fitting results are${T}_{1/2,\text{g}}\left(p\right)=322+0.046\,2p$ and${T}_{\mathrm{e}\mathrm{l},x}\left(p\right)=398+0.030\,2p$ , where the unit of temperature is K, and the unit of pressure is MPa. The smaller slope of Tel,x(p), compared with that of$ {T}_{1/2,\text{g}} $ (p), induces the temperature range (Tel,x−$ {T}_{1/2,\text{g}} $ ) in the supercooled liquid region to be narrower with the increase of pressure. DTA data in the pressure range of 2000−4500 MPa was performed by using a cubic press. A slope change in Tx(p) curve is found. Tx increases with the increasing pressure within 0.1−1700 MPa, and the rate slows down when the pressure is above 2000 MPa. In the previous diamond anvil reports, a similar pressure dependence of Tx and Tg was observed, i.e., Tx and Tg both increase initially with the increasing pressure, and then become nearly constant above 1000 MPa. Since the slope changes in Tg(p) and Tx(p) curves occur nearly at the same pressure when the microstructure of a-Se changes, in view of the pressure of 2000 MPa corresponding to the inflection point of Tx(p) curve obtained in this study, it is speculated that the pressure of the inflection point of Tg(p) curve may be around 2000 MPa. The different pressures corresponding to the slope change obtained by the diamond anvil cell and the large-volume press may be related to the measurement method of Tg and Tx, as well as the pressure measurement error.-
Key words:
- glass transition /
- amorphous selenium /
- high pressure /
- differential thermal analysis
-
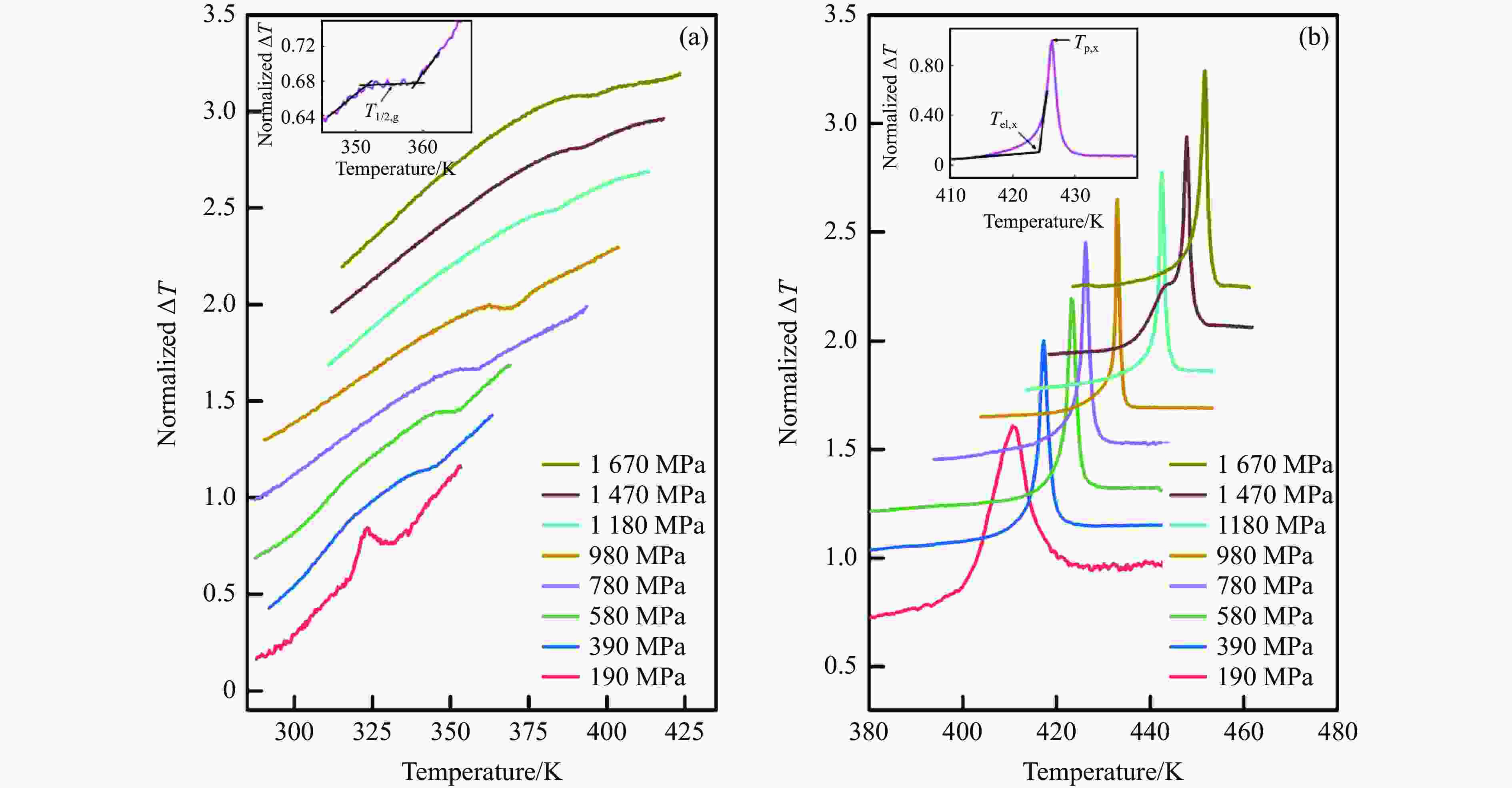
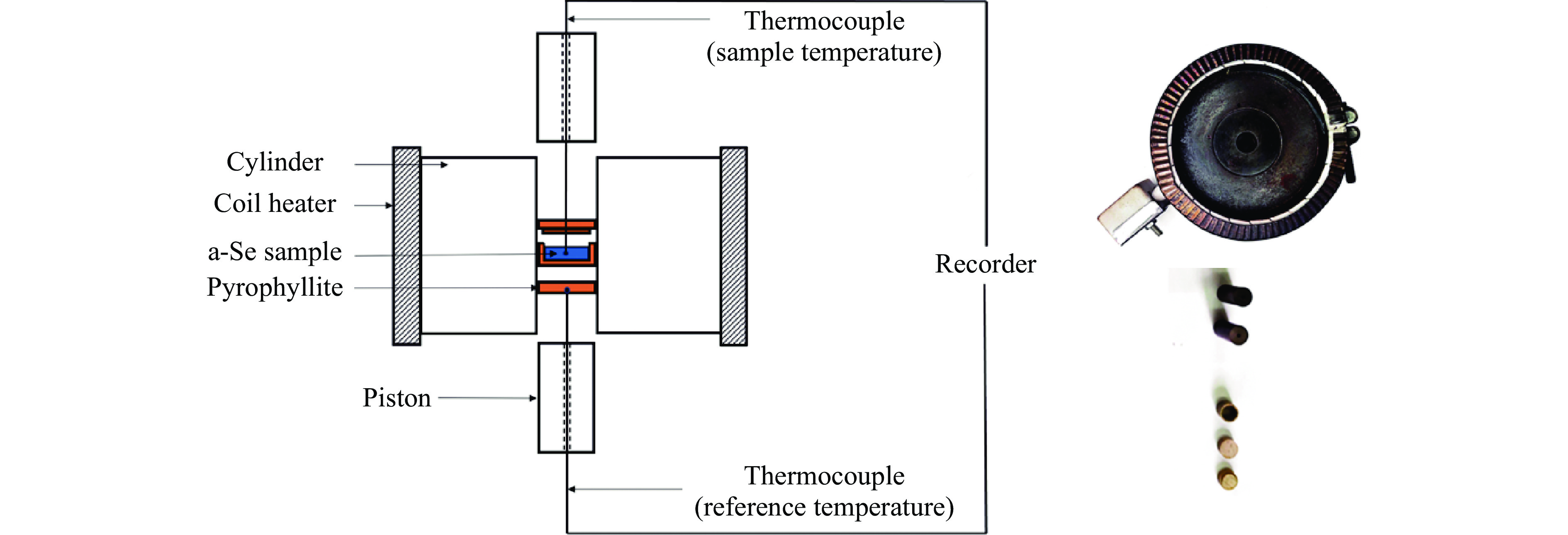
图 5 活塞圆筒装置上测得的非晶硒在不同压力下(a)玻璃化转变过程中的DTA曲线和(b)晶化过程中的DTA曲线(内插图给出了T1/2,g, Tel,x 和 Tp,x的选取方法示意图)
Figure 5. Typical DTA curves of a-Se during glass transition process (a) and crystallization process (b) under different pressures measured by using piston-cylinder apparatus (Inset figure is the determination method diagram of T1/2,g, Tel,x and Tp,x)
-
[1] 汪卫华. 非晶态物质的本质和特性 [J]. 物理学进展, 2013, 33(5): 177–351.WANG W H. The nature and properties of amorphous matter [J]. Progress in Physics, 2013, 33(5): 177–351. [2] 毛自力, 陈红, 王文魁. 高压下Zr60Ni20Al20金属玻璃形成过程的研究 [J]. 高压物理学报, 1992, 6(3): 212–216. doi: 10.11858/gywlxb.1992.03.008MAO Z L, CHEN H, WANG W K. Formation of bulk metallic glass Zr60Ni20Al20 by high pressure quenching [J]. Chinese Journal of High Pressure Physics, 1992, 6(3): 212–216. doi: 10.11858/gywlxb.1992.03.008 [3] HUANG Y N, WANG C J, RIANDE E. Superdipole liquid scenario for the dielectric primary relaxation in supercooled polar liquids [J]. The Journal of Chemical Physics, 2005, 122(14): 144502. doi: 10.1063/1.1872773 [4] CAPRION D, SCHOBER H R. Influence of the quench rate and the pressure on the glass transition temperature in selenium [J]. The Journal of Chemical Physics, 2002, 117(6): 2814–2818. doi: 10.1063/1.1492797 [5] DROZD-RZOSKA A. Pressure dependence of the glass temperature in supercooled liquids [J]. Physical Review E, 2005, 72(4): 041505. doi: 10.1103/PhysRevE.72.041505 [6] DONG Z, FRIED J R. Statistical thermodynamics of the glass transition: 1. effect of pressure and diluent concentration [J]. Computational and Theoretical Polymer Science, 1997, 7(1): 53–64. doi: 10.1016/S1089-3156(97)00008-1 [7] VLEESHOUWERS S, NIES E. Stochastic theory for the glassy state [J]. Colloid and Polymer Science, 1996, 274(2): 105–111. doi: 10.1007/BF00663442 [8] LI G, KING JR H E, OLIVER W F, et al. Pressure and temperature dependence of glass-transition dynamics in a “Fragile” glass former [J]. Physical Review Letters, 1995, 74(12): 2280–2283. doi: 10.1103/PhysRevLett.74.2280 [9] KUTCHEROV V, BÄCKSTRÖM G, ANISIMOV M, et al. Glass transition in crude oil under pressure detected by the transient hot-wire method [J]. International Journal of Thermophysics, 1993, 14(1): 91–100. doi: 10.1007/BF00522664 [10] KUTCHEROV V, LUNDIN A, ROSS R G, et al. Glass transition in viscous crude oils under pressure [J]. International Journal of Thermophysics, 1994, 15(1): 165–176. doi: 10.1007/BF01439253 [11] WILLIAMS E, ANGELL C A. Pressure dependence of the glass transition temperature in ionic liquids and solutions. evidence against free volume theories [J]. The Journal of Physical Chemistry, 1977, 81(3): 232–237. doi: 10.1021/j100518a010 [12] RZOSKA S J. New challenges for the pressure evolution of the glass temperature [J]. Frontiers in Materials, 2017, 4: 33. doi: 10.3389/fmats.2017.00033 [13] SANCHEZ I C. Towards a theory of viscosity for glass-forming liquids [J]. Journal of Applied Physics, 1974, 45(10): 4204–4215. doi: 10.1063/1.1663037 [14] JOINER B A, THOMPSON J C. Glass transition temperature shift under pressure for some semiconducting glasses [J]. Journal of Non-Crystalline Solids, 1976, 21(2): 215–224. doi: 10.1016/0022-3093(76)90042-9 [15] MI Y L, ZHENG S X. A new study of glass transition of polymers by high pressure DSC [J]. Polymer, 1998, 39(16): 3709–3712. doi: 10.1016/S0032-3861(97)10357-3 [16] SCHNEIDER H A, RUDOLF B, KARLOU K, et al. Pressure influence on the glass transition of polymers and polymer blends [J]. Polymer Bulletin, 1994, 32(5): 645–652. [17] TORATANI H, TAKAMIZAWA K. Effect of pressure on the relation between glass transition temperature and molecular weight for monodispersed polystyrenes [J]. Polymer Journal, 1994, 26(7): 797–803. doi: 10.1295/polymj.26.797 [18] EISENBERG A. The multi-dimensional glass transition [J]. The Journal of Physical Chemistry, 1963, 67(6): 1333–1336. doi: 10.1021/j100800a040 [19] YE F, LU K. Pressure effect on polymorphous crystallization kinetics in amorphous selenium [J]. Acta Materialia, 1998, 46(16): 5965–5971. doi: 10.1016/S1359-6454(98)00240-7 [20] HE Z, LIU X R, ZHANG D D, et al. Pressure effect on thermal-induced crystallization of amorphous selenium up to 5.5 GPa [J]. Solid State Communications, 2014, 197: 30–33. doi: 10.1016/j.ssc.2014.08.001 [21] BRIDGMAN P W. Compressions and polymorphic transitions of seventeen elements to 100 000 kg/cm2 [J]. Physical Review, 1941, 60(4): 351–354. doi: 10.1103/PhysRev.60.351 [22] HE Z, WANG Z G, ZHU H Y, et al. High-pressure behavior of amorphous selenium from ultrasonic measurements and Raman spectroscopy [J]. Applied Physics Letters, 2014, 105(1): 011901. doi: 10.1063/1.4887005 [23] LIU H Z, WANG L H, XIAO X H, et al. Anomalous high-pressure behavior of amorphous selenium from synchrotron X-ray diffraction and microtomography [J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(36): 13229–13234. [24] TANAKA K. Structural studies of amorphous Se under pressure [J]. Physical Review B, 1990, 42(17): 11245–11251. doi: 10.1103/PhysRevB.42.11245 [25] GUPTA M C, RUOFF A L. Transition in amorphous selenium under high pressure [J]. Journal of Applied Physics, 1978, 49(12): 5880–5884. doi: 10.1063/1.324552 [26] BERG J I, SIMHA R. Pressure-volume-temperature relations in liquid and glassy selenium [J]. Journal of Non-Crystalline Solids, 1976, 22(1): 1–22. doi: 10.1016/0022-3093(76)90002-8 [27] FORD P J, SAUNDERS G A, LAMBSON E F, et al. Investigation of the pressure dependence of the elastic constants of amorphous selenium in the vicinity of the glass-transition [J]. Philosophical Magazine Letters, 1988, 57(3): 201–206. doi: 10.1080/09500838808203772 [28] TANAKA K. Configurational and structural models for photodarkening in glassy chalcogenides [J]. Japanese Journal of Applied Physics, 1986, 25(6R): 779–786. doi: 10.1143/JJAP.25.779 [29] YAN X Z, REN X T, HE D W. Pressure calibration in solid pressure transmitting medium in large volume press [J]. Review of Scientific Instruments, 2016, 87(12): 125006. doi: 10.1063/1.4973448 [30] 王路, 王菊, 李娜娜, 等. 快速加压引起的硒熔体结晶行为 [J]. 物理学报, 2021, 70(15): 156201. doi: 10.7498/aps.70.20210253WANG L, WANG J, LI N N, et al. Mechanism of rapid compression-induced melt crystallization in selenium [J]. Acta Physica Sinica, 2021, 70(15): 156201. doi: 10.7498/aps.70.20210253 -
















 下载:
下载: