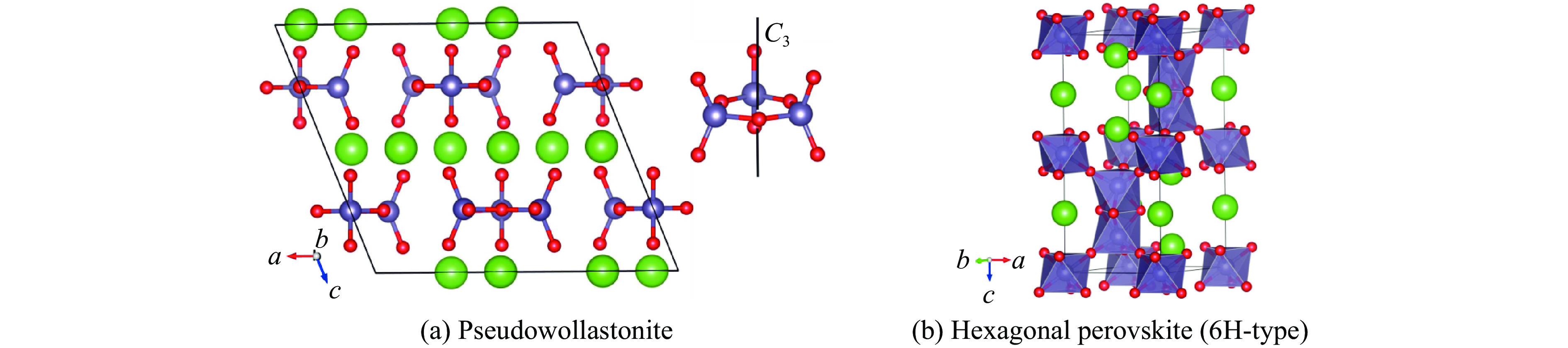
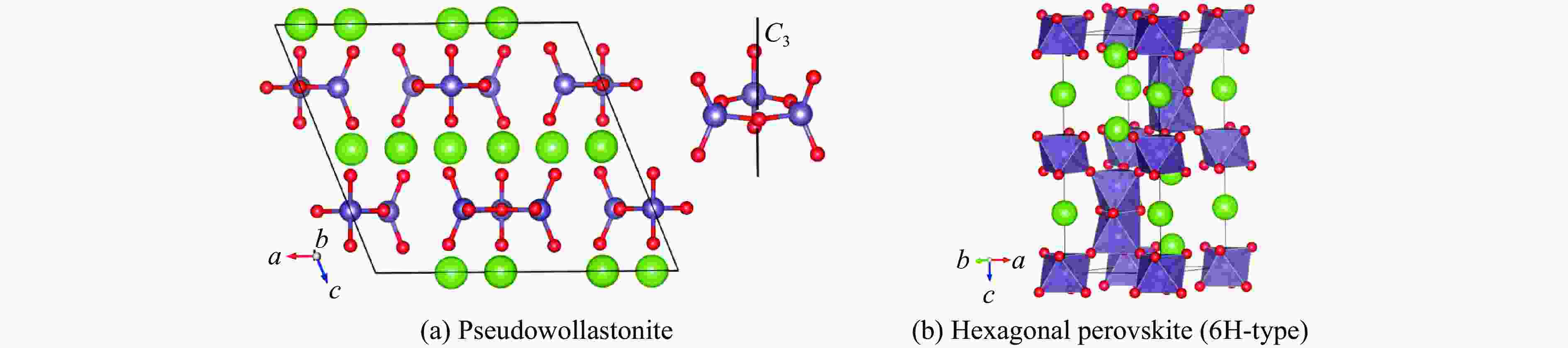
Synthesis of 6H-Type Hexagonal Perovskite Phase of BaGeO3 at High Temperature and High Pressure
-
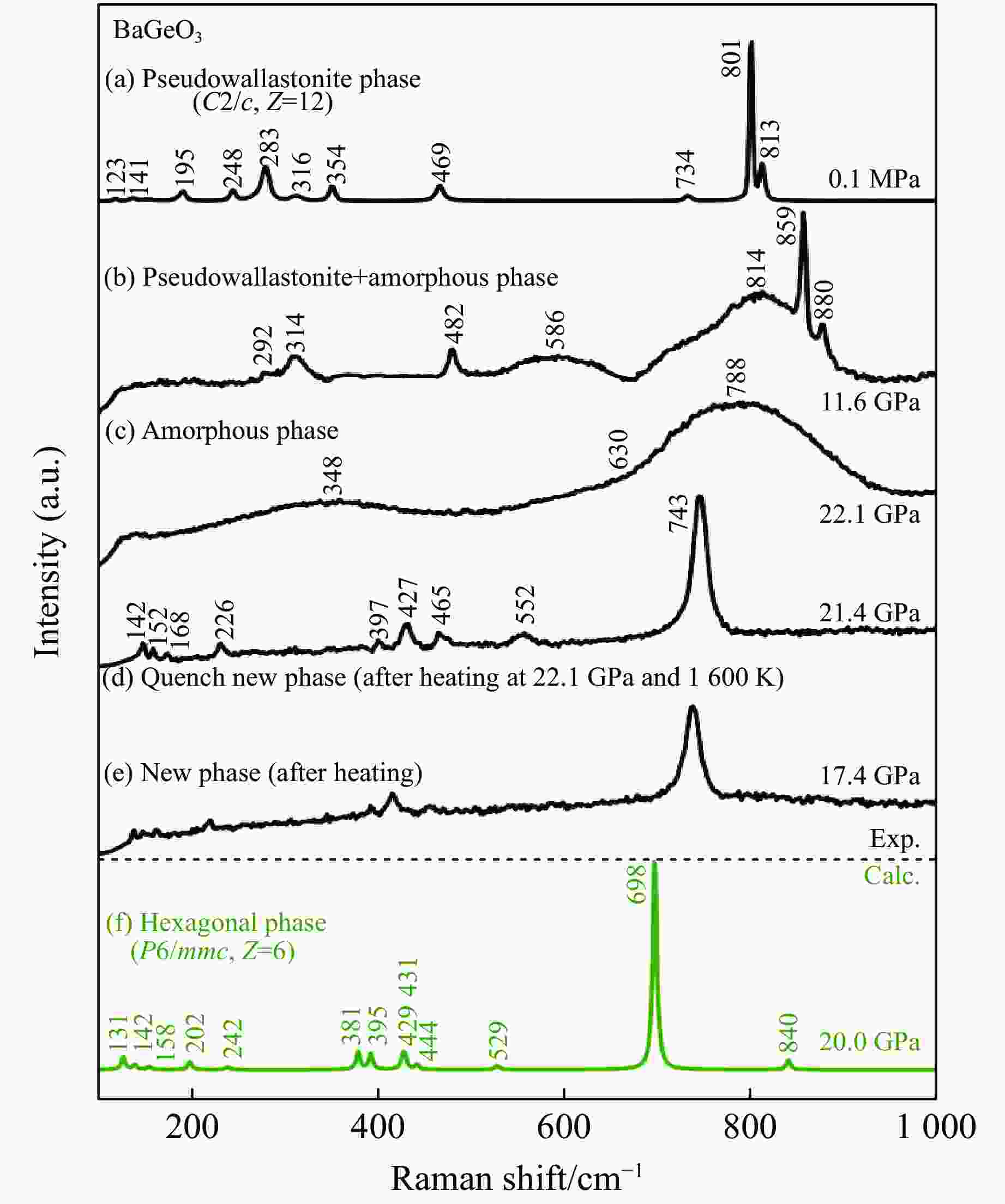
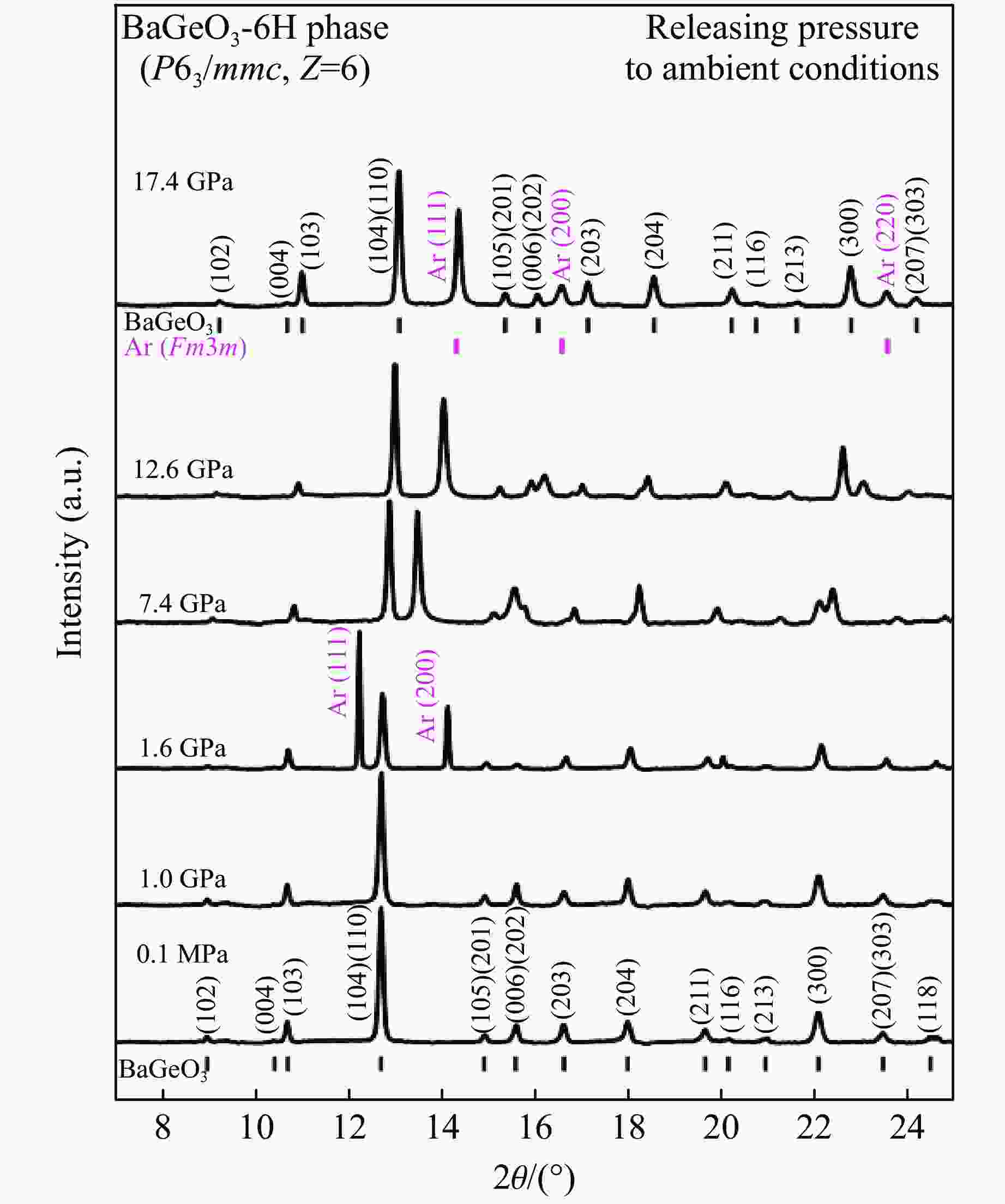
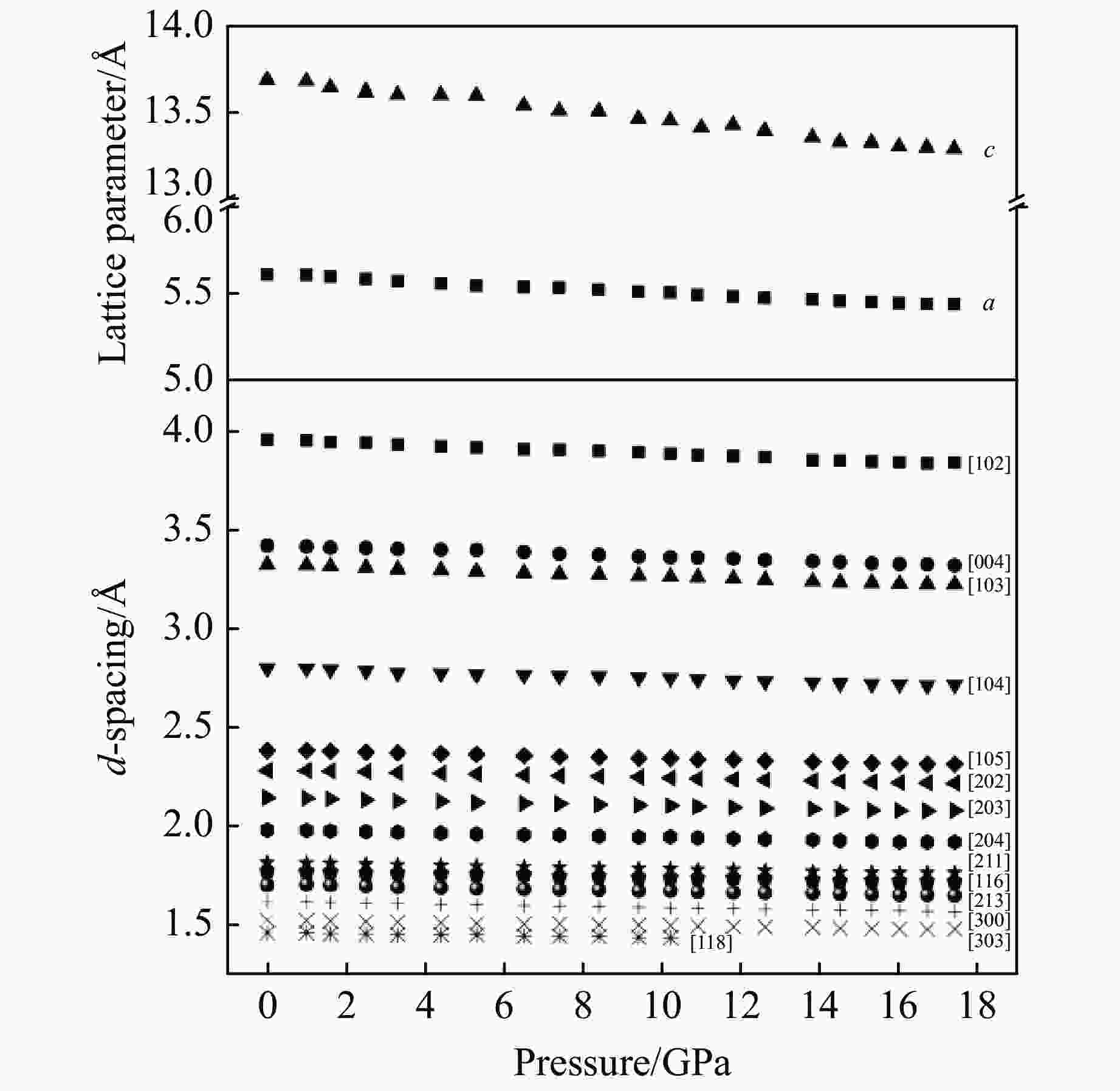
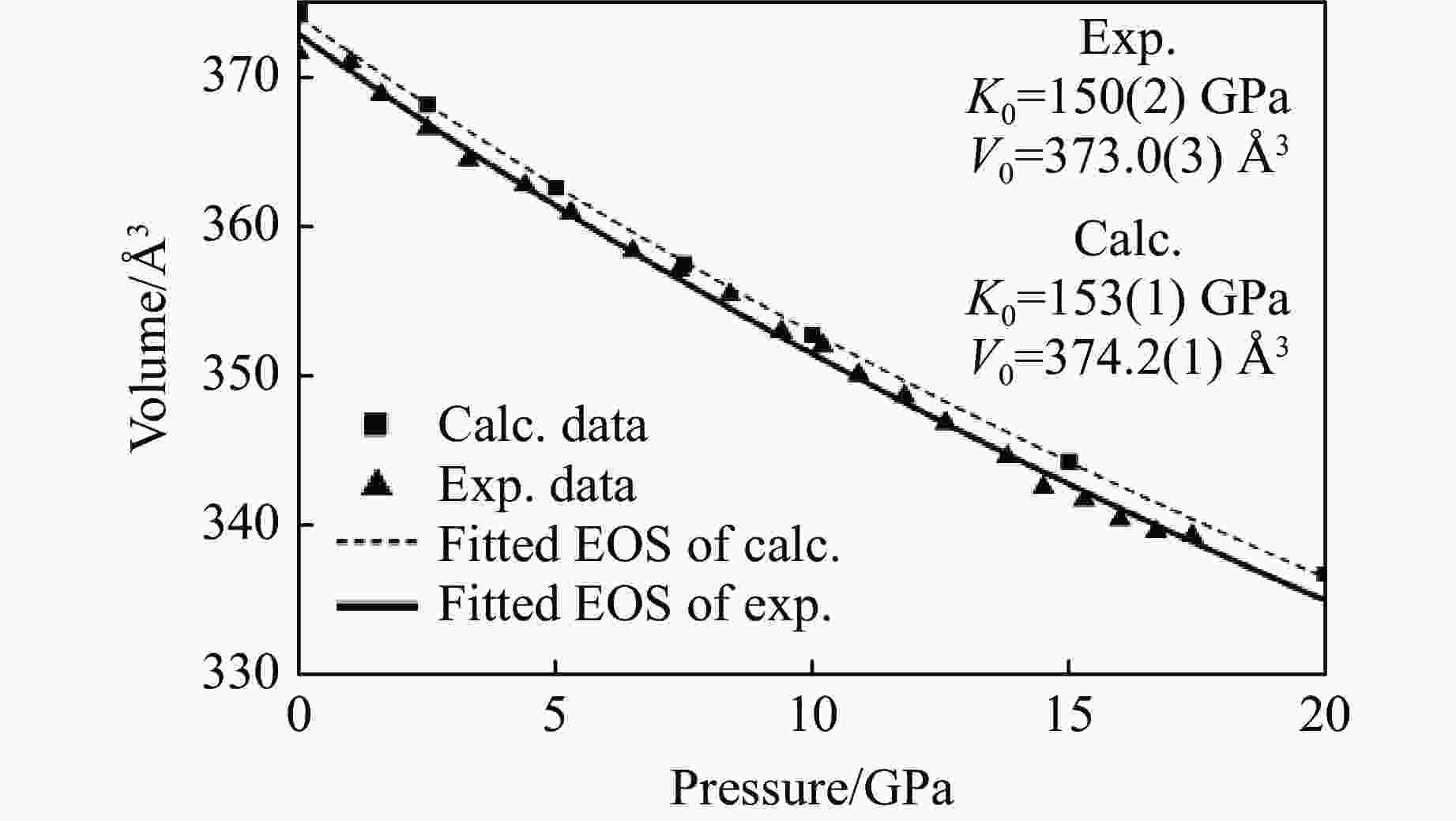
摘要: 利用金刚石对顶砧高压装置,结合显微激光双面加热技术,对BaGeO3开展了高温高压实验研究。常温常压下赝硅灰石相的BaGeO3于12 GPa左右开始非晶化。进一步加压到22 GPa并对已完全非晶化的BaGeO3样品进行(1800 ± 200) K的高温处理,拉曼光谱显示其转变成一种未见报道的高压新相。在0~17.4 GPa压力范围对BaGeO3高压新相开展同步辐射X射线衍射测试,其衍射谱可以用6H型六方钙钛矿相进行指标化,并且卸压到常压时仍保持稳定。以6H型钙钛矿相为结构模型,分别对17.4 GPa和常压下的X射线衍射谱进行Rietveld结构精修,获得其结构参数。应用二阶Birch-Murnaghan状态方程拟合实验体积-压力数据,得到其体弹模量K0 = 150(2) GPa和零压晶胞体积V0 = 373.0(3) Å3。在实验研究的基础上,对6H型钙钛矿相BaGeO3进行第一性原理理论计算,所得不同压力下的晶格常数和体积数据与实验结果符合得很好,状态方程参数K0 = 153(1) GPa,V0 = 374.2(1) Å3。20.0 GPa时计算的拉曼光谱也很好地描述了拉曼实验测量结果。研究结果补充了赝硅灰石相BaGeO3在更高温压条件下的结构相转变。6H型钙钛矿相BaGeO3的获得为进一步表征该相的物理化学性质奠定了基础,为开发高性能钙钛矿结构锗酸盐材料提供了可能性,同时对于理解硅酸盐钙钛矿结构的相变规律及稳定性、地球下地幔物理化学性质及其变化等具有重要的指示意义。
-
关键词:
- BaGeO3 /
- 6H型六方钙钛矿相 /
- Rietveld结构精修 /
- 拉曼光谱 /
- 高温高压
Abstract: Barium germanate (BaGeO3) was studied using double-sided laser-heating diamond anvil cell (LHDAC). At ambient conditions, BaGeO3 has a pseudowollastonite structure. At about 12 GPa, BaGeO3 crystal begin to translate into an amorphous phase. The amorphous BaGeO3 was further pressurized to about 22 GPa and then heated at (1800 ± 200) K conditions. Raman spectra shows the amorphous BaGeO3 transforms into a new high pressure phase, which has not been reported so far. The new high pressure phase of BaGeO3 was further measured with the synchrotron radiation X-ray diffraction in the pressure ranges of 0−17.4 GPa. The diffraction patterns can be indexed with a 6H-type hexagonal perovskite structure and this structure remained stable as the pressure unloading to ambient pressure. In order to obtain the structural parameters of the new high pressure phase of BaGeO3, the X-ray diffraction patterns of 17.4 GPa and ambient pressure were refined with a model structure of 6H-type perovskite using the Rietveld method. The experimental pressure-volume data was fitted with the second-order Birch-Murnaghan equation of state, and obtained the volume bulk modulus and zero-pressure unit-cell volume are K0 = 150(2) GPa and V0 = 373.0(3) A3 respectively. On the basis of the experimental results in this study, we also carried out the first-principle theoretical calculation on the 6H-type perovskite BaGeO3. The calculated lattice constants and volume with the corresponding pressures are good agreement with the experimental results. Furthermore, the calculated volume bulk modulus and zero-pressure unit-cell volume are K0 = 153(1) GPa, V0 = 374.2(1) A3 respectively. The calculated Raman spectra at 20.0 GPa is also well consistent with the experimental results. This study not only complements the structural phase transition of pseudowallastonite BaGeO3 at high temperature and high pressure, but also builds a solid foundation for further characterizing the physical and chemical properties of pseudowallastonite BaGeO3, and gives a chance to develop the perovskite structured germanate functional materials. In addition, this study has an important indicative significance for us to understand the phase transition rule and stability of silicate perovskite, the physical and chemical properties and changes of Earth's lower mantle. -
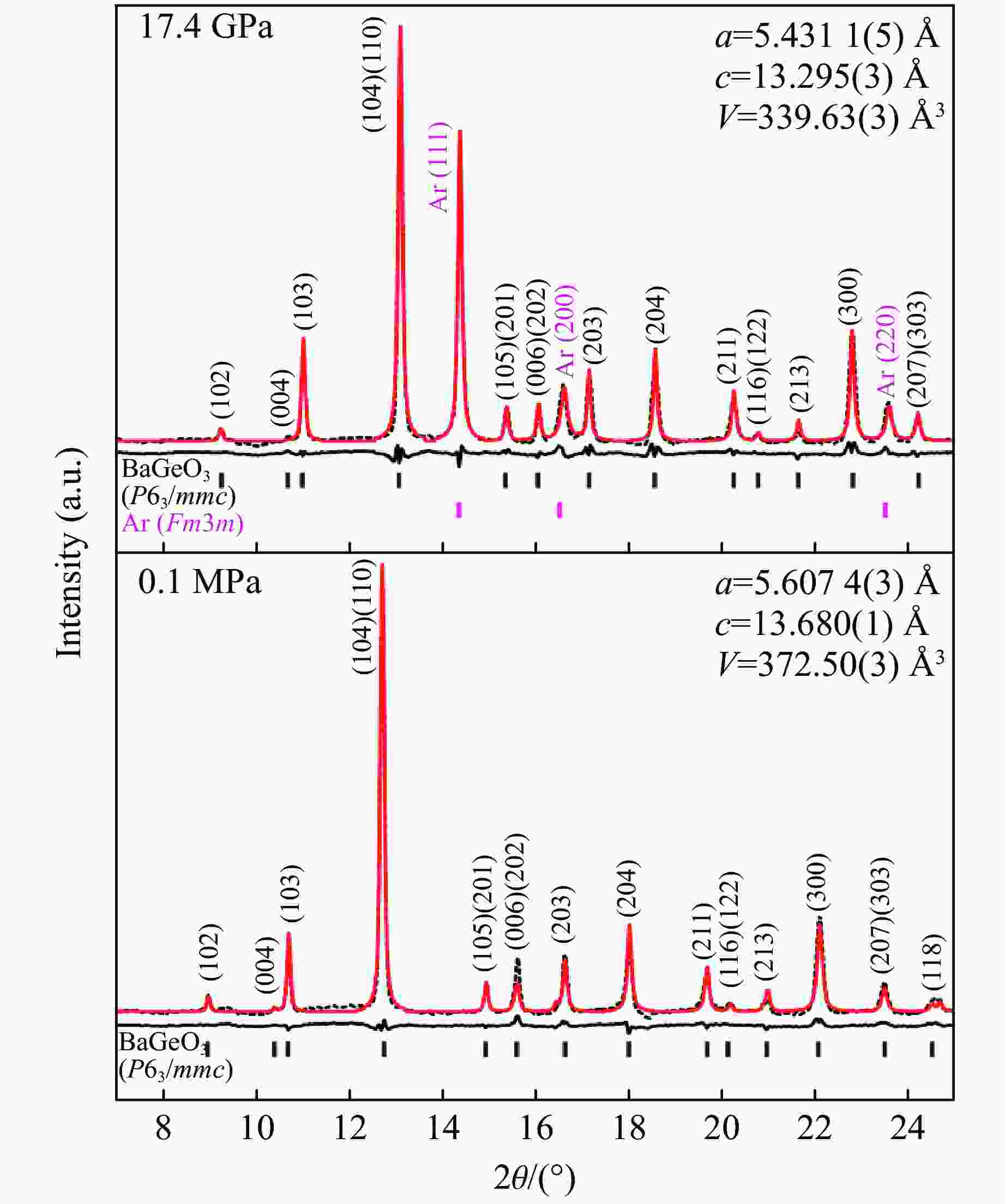
表 1 6H型六方钙钛矿相BaGeO3在常压和17.4 GPa的结构参数
Table 1. Structural parameters of hexagonal perovskite phase BaGeO3 (6H-type) at ambient pressure and 17.4 GPa
Structural parameters at 17.4 GPa Structural parameters at 0.1 MPa Atom Site x y z Atom Site x y z Ba1 2b 0 0 0.2500 Ba1 2b 0 0 0.2500 Ba2 4f 0.3333 0.6667 0.0896(5) Ba2 4f 0.3333 0.6667 0.0930(5) Ge1 2a 0 0 0 Ge1 2a 0 0 0 Ge2 4f 0.3333 0.6667 0.8392(5) Ge2 4f 0.3333 0.6667 0.8427(5) O1 6h 0.5178(27) 0.0360(5) 0.2500 O1 6h 0.4976(23) −0.0050(5) 0.2500 O2 12k 0.8342(27) 0.6680(5) 0.0730(5) O2 12k 0.8140(23) 0.6280 0.0765 Bond lengths at 17.4 GPa/Å Bond lengths at 0.1 MPa/Å Ge1―O2 Ge2―O2 Ge2―O1 Ge1―O2 Ge2―O2 Ge2―O1 1.837(19) × 6 1.961(16) × 3 1.835(17) × 3 2.088(17) × 6 1.808(14) × 3 2.075(15) × 3 Note: Numbers in parentheses indicate standard deviation. 表 2 计算得到的6H型六方钙钛矿相BaGeO3在20.0 GPa下的拉曼振动模
Table 2. Calculated Raman vibrational modes of hexagonal perovskite phase BaGeO3 (6H-type) at 20.0 GPa cm−1
E2g E1g A1g E2g E1g E2g E2g E1g A1g E1g 88 131 142 158 202 213 242 264 381 395 E2g E2g E1g A1g E2g E2g E1g A1g A1g 399 429 431 444 529 576 578 698 840 -
[1] MIZOGUCHI H, KAMIYA T, MATSUISHI S, et al. A germanate transparent conductive oxide [J]. Nature Communications, 2011, 2: 470. doi: 10.1038/NCOMMS1484 [2] HORIUCHI H, ITO E, WEIDNER D J. Perovskite-type MgSiO3: single-crystal X-ray diffraction study [J]. American Mineralogist, 1987, 72(3/4): 357–360. [3] MAO H K, CHEN L C, HEMLEY R J, et al. Stability and equation of state of CaSiO3-perovskite to 134 GPa [J]. Journal of Geophysical Research: Solid Earth, 1989, 94(B12): 17889–17894. doi: 10.1029/JB094iB12p17889 [4] XIAO W S, TAN D Y, ZHOU W, et al. A new cubic perovskite in PbGeO3 at high pressures [J]. American Mineralogist, 2012, 97(7): 1193–1198. doi: 10.2138/am.2012.4021 [5] XIAO W S, TAN D Y, ZHOU W, et al. Cubic perovskite polymorph of strontium metasilicate at high pressures [J]. American Mineralogist, 2013, 98(11/12): 2096–2104. doi: 10.2138/am.2013.4470 [6] GIBBS G V, BOISEN M B, HILL F C, et al. SiO and GeO bonded interactions as inferred from the bond critical point properties of electron density distributions [J]. Physics and Chemistry of Minerals, 1998, 25(8): 574–584. doi: 10.1007/s002690050150 [7] AKAOGI M, KOJITANI H, YUSA H, et al. High-pressure transitions and thermochemistry of MGeO3 (M = Mg, Zn and Sr) and Sr-silicates: systematics in enthalpies of formation of A2+B4+O3 perovskites [J]. Physics and Chemistry of Minerals, 2005, 32(8/9): 603–613. doi: 10.1007/s00269-005-0034-1 [8] NAKATSUKA A, ARIMA H, OHTAKA O, et al. Crystal structure of SrGeO3 in the high-pressure perovskite-type phase [J]. Acta Crystallographica Section E: Crystallographic Communications, 2015, 71(5): 502–504. doi: 10.1107/S2056989015007264 [9] ROSS N L, ANGEL R J. Compression of CaTiO3 and CaGeO3 perovskites [J]. American Mineralogist, 1999, 84(3): 277–281. doi: 10.2138/am-1999-0309 [10] RUNGE C E, KUBO A, KIEFER B, et al. Equation of state of MgGeO3 perovskite to 65 GPa: comparison with the post-perovskite phase [J]. Physics and Chemistry of Minerals, 2006, 33(10): 699–709. doi: 10.1007/s00269-006-0116-8 [11] YUSA H, AKAOGI M, SATA N, et al. Letter: unquenchable hexagonal perovskite in high-pressure polymorphs of strontium silicates [J]. American Mineralogist, 2005, 90(5/6): 1017–1020. doi: 10.2138/am.2005.1835 [12] YUSA H, SATA N, OHISHI Y. Rhombohedral (9R) and hexagonal (6H) perovskites in barium silicates under high pressure [J]. American Mineralogist, 2007, 92(4): 648–654. doi: 10.2138/am.2007.2314 [13] HIRAMATSU H, YUSA H, IGARASHI R, et al. An exceptionally narrow band-gap (~4 eV) silicate predicted in the cubic perovskite structure: BaSiO3 [J]. Inorganic Chemistry, 2017, 56(17): 10535–10542. doi: 10.1021/acs.inorgchem.7b01510 [14] YANG H X, PREWITT C T. Crystal structure and compressibility of a two-layer polytype of pseudowollastonite (CaSiO3) [J]. American Mineralogist, 1999, 84(11/12): 1902–1905. doi: 10.2138/am-1999-11-1217 [15] NISHI F. Strontium metagermanate, SrGeO3 [J]. Acta Crystallographica Section C: Crystal Structure Communications, 1997, 53(4): 399–401. doi: 10.1107/S0108270196013960 [16] WAN S M, ZENG Y, YAO Y N, et al. BaGeO3: a mid-IR transparent crystal with superstrong raman response [J]. Inorganic Chemistry, 2020, 59(6): 3542–3545. doi: 10.1021/acs.inorgchem.0c00155 [17] GSPAN C, KAHLENBERG V, KOTHLEITNER G, et al. Atomic and domain structure of the low-temperature phase of barium metagermanate (BaGeO3) [J]. Acta Crystallographica Section A: Foundations of Crystallography, 2006, 62(6): 1002–1009. doi: 10.1107/S0108768106039140 [18] SHIMIZU Y, SYONO Y, AKIMOTO S. High-pressure transformations in SrGeO3, SrSiO3, BaGeO3, and BaSiO3 [J]. High Temperatures-High Pressures, 1970, 2(1): 113–120. [19] OZIMA M, SUSAKI J I, AKIMOTO S I, et al. The system BaO-GeO2 at high pressures and temperatures, with special reference to high-pressure transformations in BaGeO3, BaGe2O5, and Ba2Ge5O12 [J]. Journal of Solid State Chemistry, 1982, 44(3): 307–317. doi: 10.1016/0022-4596(82)90378-4 [20] GASPARIK T, WOLF K, SMITH C M. Experimental determination of phase relations in the CaSiO3 system from 8 to 15 GPa [J]. American Mineralogist, 1994, 79(11/12): 1219–1222. [21] AKAOGI M, YANO M, TEJIMA Y, et al. High-pressure transitions of diopside and wollastonite: phase equilibria and thermochemistry of CaMgSi2O6, CaSiO3 and CaSi2O5-CaTiSiO5 system [J]. Physics of the Earth and Planetary Interiors, 2004, 143/144: 145–156. doi: 10.1016/j.pepi.2003.08.008 [22] KATZ L, WARD R. Structure relations in mixed metal oxides [J]. Inorganic Chemistry, 1964, 3(2): 205–211. doi: 10.1021/ic50012a013 [23] CHENG J G, ALONSO J A, SUARD E, et al. A new perovskite polytype in the high-pressure sequence of BaIrO3 [J]. Journal of the American Chemical Society, 2009, 131(21): 7461–7469. doi: 10.1021/ja901829e [24] SASAKI S, PREWITT C T, LIEBERMANN R C. The crystal structure of CaGeO3 perovskite and the crystal chemistry of the GdFeO3-type perovskites [J]. American Mineralogist, 1983, 68(11/12): 1189–1198. [25] SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides [J]. Acta Crystallographica Section A: Foundations and Advances, 1976, 32(5): 751–767. doi: 10.1107/S0567739476001551 [26] MAO H K, XU J, BELL P M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions [J]. Journal of Geophysical Research: Solid Earth, 1986, 91(B5): 4673–4676. doi: 10.1029/JB091iB05p04673 [27] HAMMERSLEY A P, SVENSSON S O, HANFLAND M, et al. Two-dimensional detector software: from real detector to idealised image or two-theta scan [J]. High Pressure Research, 1996, 14(4/6): 235–248. doi: 10.1080/08957959608201408 [28] HOLLAND T J B, REDFERN S A T. Unit cell refinement from powder diffraction data: the use of regression diagnostics [J]. Mineralogical Magazine, 1997, 61(404): 65–77. doi: 10.1180/MINMAG.1997.061.404.07 [29] TOBY B H, VON DREELE R B. GSAS-Ⅱ: the genesis of a modern open-source all purpose crystallography software package [J]. Journal of Applied Crystallography, 2013, 46(2): 544–549. doi: 10.1107/S0021889813003531 [30] GONZALEZ-PLATAS J, ALVARO M, NESTOLA F, et al. EosFit7-GUI: a new graphical user interface for equation of state calculations, analyses and teaching [J]. Journal of Applied Crystallography, 2016, 49(4): 1377–1382. doi: 10.1107/S1600576716008050 [31] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple [J]. Physical Review Letters, 1996, 77(18): 3865–3868. doi: 10.1103/PhysRevLett.77.3865 [32] LIN C C, SHEN P Y. Pressure-induced metastable phase transformations of calcium metasilicate (CaSiO3): a Raman spectroscopic study [J]. Materials Chemistry and Physics, 2016, 182: 508–519. doi: 10.1016/j.matchemphys.2016.07.065 [33] KRONBO C H, MENESCARDI F, CERESOLI D, et al. High pressure structure studies of three SrGeO3 polymorphs: amorphization under pressure [J]. Journal of Alloys and Compounds, 2021, 855: 157419. doi: 10.1016/j.jallcom.2020.157419 -






 下载:
下载: