Pressure-Induced Metallization and Novel Superconductivity of Chalcogen Hydrides
-
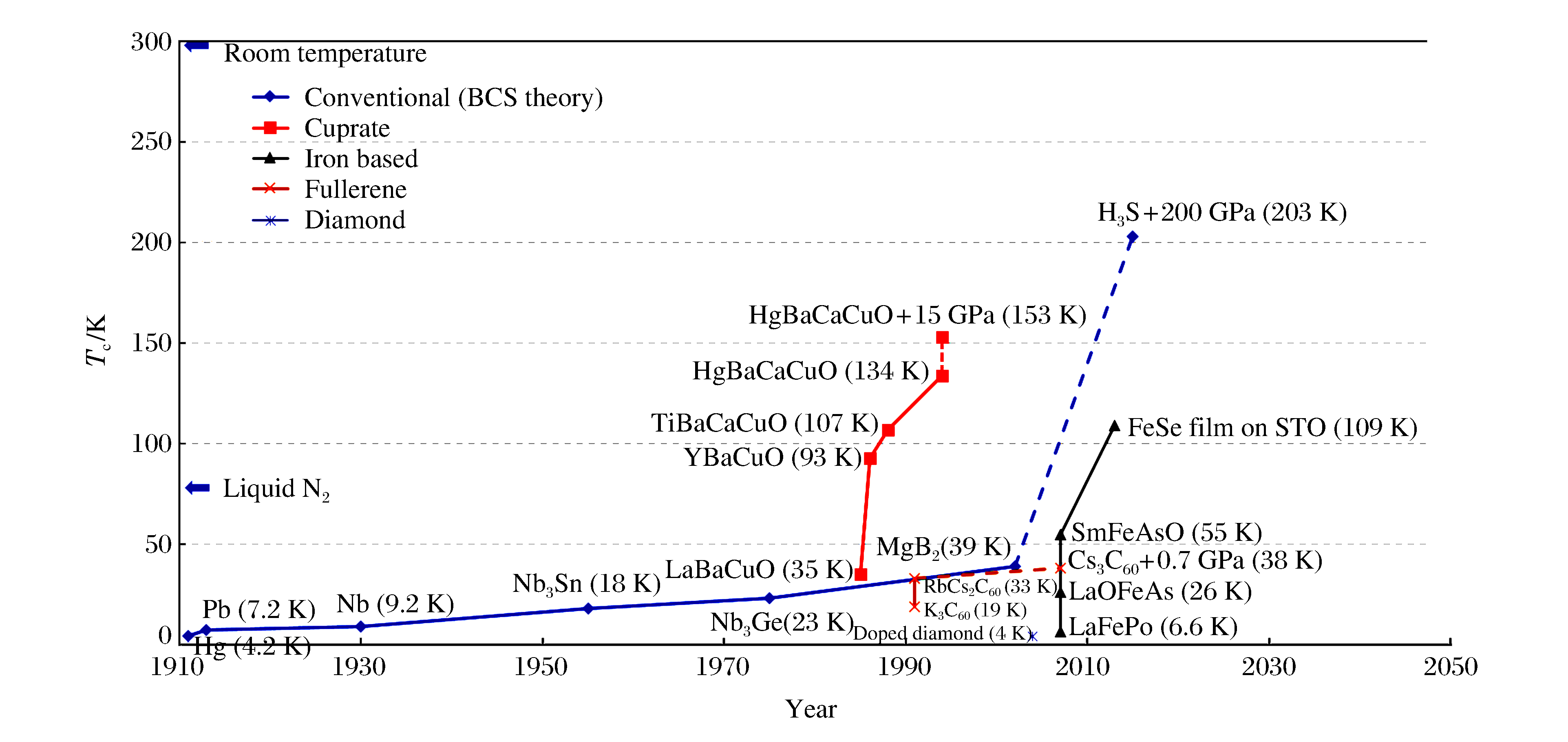
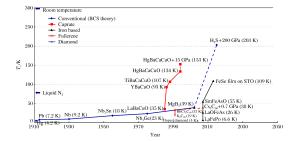
摘要: 富氢化合物在目前实验所能达到的压力范围内有望实现金属化,是潜在的具有高超导临界温度的材料。实验和理论研究均发现高压下S-H化合物的超导临界温度高达203 K,创造了高温超导的新纪录,掀起了新一轮富氢化合物超导电性研究的热潮。本文主要介绍近年来关于氧族氢化物的压致金属化和奇异超导电性研究,对比分析氧族富氢化合物高压行为的异同。氧族元素的最外层电子排布相同,但原子质量和电负性的差异巨大,导致形成的富氢化合物在化学配比、结构、化学成键以及超导电性来源上存在较大差别。S-H和Se-H化合物的超导电性主要源自与氢原子拉伸振动模式相关的强电子-声子耦合,而Te-H和Po-H化合物中对超导电性贡献最大的是氢原子的切向振动模式。Abstract: Owing to their expected capability to reach metallization within the pressure range under the present laboratory conditions, hydrogen-rich compounds are considered promising candidates for potential high-Tc (superconductor critical temperature) superconductors.Both experimental and theoretical research have found that the critical high-temperature superconductivity can reach a record high-Tc as up to 203 K in compressed sulfur hydrides, thereby generating a new wave for searching for new hydrogen-rich superconductors.The present review focuses on researches of pressure-induced metallization and novel superconductivity in chalcogen hydrides, and discusses their differences in structures and various physical and chemical properties.Chalcogen atoms are isoelectronic but differ a lot in atomic mass and electronegativity, resulting in their great differences in stoichiometry, structure, and chemical bonding.The high-Tc superconductivity of Te/Po-H compounds originates from the strong electron-phonon couplings associated with the intermediate frequency of H-derived wagging and bending modes, a superconducting mechanism which differs substantially from those in S/Se-H compounds where the high frequency H-stretching vibrations make considerable contributions.
-
Key words:
- high pressure /
- chalcogen hydrides /
- crystal structure /
- superconductivity
-
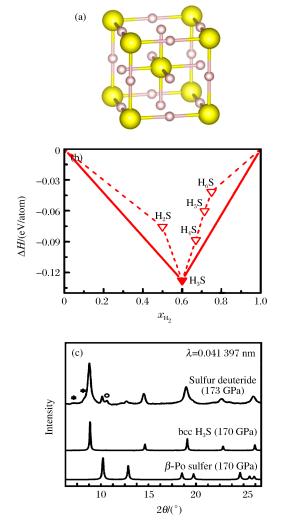
图 2 (a) Im-3m相H3S的晶体结构,小球表示H原子;(b) 150 GPa压力下HxS (x=2, 3, 4, 5, 6)化合物相对于单质H2和单质S分解的形成焓凸包图[17];(c)不包含靶材特征峰的压致H2S的XRD谱[67](图中显示了H3S的Im-3m相和单质S的β-Po结构在170 GPa压力下的XRD谱,星号表示不属于样品的峰,空心圆标识的峰位对应高压下单质硫的第Ⅳ相)
Figure 2. (a) Crystal structure of H3S with Im-3m symmetry.The small ball indicates hydrogen.(b) Predicted formation enthalpies of HxS (x=2, 3, 4, 5, 6) with respect to decomposition into S and H2 under 150 GPa[17].(c) Integrated XRD patterns obtained with subtraction of the background for H2S.The patterns of Im-3m H3S and β-Po elemental sulfur at 170 GPa are shown in the experimentally obtained patterns.The asterisks indicate the peaks that do not belong to the sample and the open circles indicate a reflection fromthe high-pressure phase Ⅳ of the elemental sulfur[67].
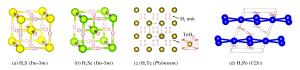
图 3 (a) 氧族氢化物S-H[17]、Se-H[19]、Te-H[26]、Po-H[27]的形成焓凸包图;(b)氧族氢化物中富含氢且具有较高Tc的化学计量比的稳定压力区间
Figure 3. (a) Formation enthalpies of various chalcogen hydrides (S-H[17], Se-H[19], Te-H[26], Po-H[27]) with respect to decomposition into constituent elemental solids under pressure; (b) Pressure ranges in which the corresponding structures of different hydrogen-rich stoichiometries with high Tc are stabilized
-
[1] ONNES H K.The resistance of pure mercury at helium temperatures[J]. Communications Physical Laboratory University of Leiden, 1911, 12(120):1. http://www.citeulike.org/user/andreassorge/article/7386247 [2] BARDEEN J, COOPER L N, SCHRIEFFER J R.Theory of superconductivity[J]. Physical Review, 1957, 108(5):1175-1204. doi: 10.1103/PhysRev.108.1175 [3] WIGNER E, HUNTINGTON H B.On the possibility of a metallic modification of hydrogen[J]. The Journal of Chemical Physics, 1935, 3(12):764-770. doi: 10.1063/1.1749590 [4] ASHCROFT N W.Metallic hydrogen:a high-temperature superconductor?[J]. Physical Review Letters, 1968, 21(26):1748-1749. doi: 10.1103/PhysRevLett.21.1748 [5] ZHANG L J, NIU Y L, LI Q, et al.Ab initio prediction of superconductivity in molecular metallic hydrogen under high pressure[J]. Solid State Communications, 2007, 141(11):610-614. doi: 10.1016/j.ssc.2006.12.029 [6] DALLADAY-SIMPSON P, HOWIE R T, GREGORYANZ E.Evidence for a new phase of dense hydrogen above 325 gigapascals[J]. Nature, 2016, 529(7584):63-67. doi: 10.1038/nature16164 [7] ASHCROFT N W.Hydrogen dominant metallic alloys:high temperature superconductors?[J]. Physical Review Letters, 2004, 92(18):187002. doi: 10.1103/PhysRevLett.92.187002 [8] LI Y, HAO J, LIU H, et al.The metallization and superconductivity of dense hydrogen sulfide[J]. The Journal of Chemical Physics, 2014, 140(17):174712. doi: 10.1063/1.4874158 [9] DROZDOV A P, EREMETS M I, TROYAN I A, et al.Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system[J]. Nature, 2015, 525(7567):73-76. doi: 10.1038/nature14964 [10] DUAN D F, LIU Y X, TIAN F B, et al.Pressure-induced metallization of dense (H2S)2H2 with high-Tc superconductivity[J]. Scientific Reports, 2014, 4:6968. https://www.researchgate.net/profile/David_Wood14 [11] BERNSTEIN N, HELLBERG C S, JOHANNES M D, et al.What superconducts in sulfur hydrides under pressure and why[J]. Physical Review B, 2015, 91(6):060511. doi: 10.1103/PhysRevB.91.060511 [12] DUAN D F, HUANG X L, TIAN F B, et al.Pressure-induced decomposition of solid hydrogen sulfide[J]. Physical Review B, 2015, 91(18):180502. doi: 10.1103/PhysRevB.91.180502 [13] ERREA I, CALANDRA M, PICKARD C J, et al.High-pressure hydrogen sulfide from first principles:a strongly anharmonic phonon-mediated superconductor[J]. Physical Review Letters, 2015, 114(15):157004. doi: 10.1103/PhysRevLett.114.157004 [14] PAPACONSTANTOPOULOS D A, KLEIN B M, MEHL M J, et al.Cubic H3S around 200 GPa:an atomic hydrogen superconductor stabilized by sulfur[J]. Physical Review B, 2015, 91(18):184511. doi: 10.1103/PhysRevB.91.184511 [15] GE Y F, ZHANG F, YAO Y G.First-principles demonstration of superconductivity at 280 K in hydrogen sulfide with low phosphorus substitution[J]. Physical Review B, 2016, 93(22):224513. doi: 10.1103/PhysRevB.93.224513 [16] ISHIKAWA T, NAKANISHI A, SHIMIZU K, et al.Superconducting H5S2 phase in sulfur-hydrogen system under high-pressure[J]. Scientific Reports, 2016, 6:23160. doi: 10.1038/srep23160 [17] LI Y W, WANG L, LIU H Y, et al.Dissociation products and structures of solid H2S at strong compression[J]. Physical Review B, 2016, 93(2):020103. http://www.osti.gov/scitech/biblio/1387308 [18] ZHANG H D, JIN X L, LV Y Z, et al.A novel stable hydrogen-rich SnH8 under high pressure[J]. RSC Advances, 2015, 5(130):107637-107641. doi: 10.1039/C5RA20428C [19] ZHANG S T, WANG Y C, ZHANG J R, et al.Phase diagram and high-temperature superconductivity of compressed selenium hydrides[J]. Scientific Reports, 2015, 5:15433. doi: 10.1038/srep15433 [20] ESFAHANI M M D, WANG Z, OGANOV A R, et al.Superconductivity of novel tin hydrides (SnnHm) under pressure[J]. Scientific Reports, 2016, 6:22873. doi: 10.1038/srep22873 [21] FLORES-LIVAS J A, AMSLER M, HEIL C, et al.Superconductivity in metastable phases of phosphorus-hydride compounds under high pressure[J]. Physical Review B, 2016, 93(2):020508. doi: 10.1103/PhysRevB.93.020508 [22] FU Y H, DU X P, ZHANG L J, et al.High-pressure phase stability and superconductivity of pnictogen hydrides and chemical trends for compressed hydrides[J]. Chemistry of Materials, 2016, 28(6):1746-1755. doi: 10.1021/acs.chemmater.5b04638 [23] KOKAIL C, HEIL C, BOERI L.Search for high-Tc conventional superconductivity at megabar pressures in the lithium-sulfur system[J]. Physical Review B, 2016, 94(6):060502. doi: 10.1103/PhysRevB.94.060502 [24] LIU Y X, DUAN D F, TIAN F B, et al.Stability and properties of the Ru-H system at high pressure[J]. Physical Chemistry Chemical Physics, 2016, 18(3):1516-1520. doi: 10.1039/C5CP06617D [25] STRUZHKIN V V, KIM D Y, STAVROU E, et al.Synthesis of sodium polyhydrides at high pressures[J]. Nature Communications, 2016, 7:12267. doi: 10.1038/ncomms12267 [26] ZHONG X, WANG H, ZHANG J R, et al.Tellurium hydrides at high pressures:high-temperature superconductors[J]. Physical Review Letters, 2016, 116(5):057002. doi: 10.1103/PhysRevLett.116.057002 [27] LIU Y X, DUAN D F, TIAN F B, et al.Prediction of stoichiometric PoHn compounds:crystal structures and properties[J]. RSC Advances, 2015, 5(125):103445-103450. doi: 10.1039/C5RA19223D [28] CHEN C B, XU Y, SUN X P, et al.Novel superconducting phases of HCl and HBr under high pressure:an ab initio study[J]. The Journal of Physical Chemistry C, 2015, 119(30):17039-17043. doi: 10.1021/acs.jpcc.5b01653 [29] CHENG Y, ZHANG C, WANG T T, et al.Pressure-induced superconductivity in H2-containing hydride PbH4(H2)2[J]. Scientific Reports, 2015, 5:16475. doi: 10.1038/srep16475 [30] FENG X L, ZHANG J R, GAO G Y, et al.Compressed sodalite-like MgH6 as a potential high-temperature superconductor[J]. RSC Advances, 2015, 5(73):59292-59296. doi: 10.1039/C5RA11459D [31] HEIL C, BOERI L.Influence of bonding on superconductivity in high-pressure hydrides[J]. Physical Review B, 2015, 92(6):060508. doi: 10.1103/PhysRevB.92.060508 [32] HOU P G, TIAN F B, LI D, et al.Ab initio study of germanium-hydride compounds under high pressure[J]. RSC Advances, 2015, 5(25):19432-19438. doi: 10.1039/C4RA13183E [33] HOU P G, ZHAO X S, TIAN F B, et al.High pressure structures and superconductivity of AlH3(H2) predicted by first principles[J]. RSC Advances, 2015, 5(7):5096-5101. doi: 10.1039/C4RA14990D [34] LI Y W, HAO J, LIU H Y, et al.Pressure-stabilized superconductive yttrium hydrides[J]. Scientific Reports, 2015, 5:9948. doi: 10.1038/srep09948 [35] LIU Y C, DUAN D F, HUANG X L, et al.Structures and properties of osmium hydrides under pressure from first principle calculation[J]. The Journal of Physical Chemistry C, 2015, 119(28):15905-15911. doi: 10.1021/acs.jpcc.5b03791 [36] LIU Y C, DUAN D F, TIAN F B, et al.Pressure-induced structures and properties in indium hydrides[J]. Inorganic Chemistry, 2015, 54(20):9924-9928. doi: 10.1021/acs.inorgchem.5b01684 [37] LIU Y C, DUAN D F, TIAN F B, et al.Prediction of stoichiometric PoHn compounds:crystal structures and properties[J]. RSC Advances, 2015, 5(125):103445-103450. doi: 10.1039/C5RA19223D [38] MURAMATSU T, WANENE W K, SOMAYAZULU M, et al.Metallization and superconductivity in the hydrogen-rich ionic salt BaReH9[J]. The Journal of Physical Chemistry C, 2015, 119(32):18007-18013. doi: 10.1021/acs.jpcc.5b03709 [39] PÉPIN C, LOUBEYRE P, OCCELLI F, et al.Synthesis of lithium polyhydrides above 130 GPa at 300 K[J]. Proceedings of the National Academy of Sciences, 2015, 112(25):7673-7676. doi: 10.1073/pnas.1507508112 [40] SHAMP A, ZUREK E.Superconducting high-pressure phases composed of hydrogen and iodine[J]. The Journal of Physical Chemistry Letters, 2015, 6(20):4067-4072. doi: 10.1021/acs.jpclett.5b01839 [41] WANG Y C, WANG H, JOHN S T, et al.Structural morphologies of high-pressure polymorphs of strontium hydrides[J]. Physical Chemistry Chemical Physics, 2015, 17(29):19379-19385. doi: 10.1039/C5CP01510C [42] YU S Y, JIA X J, FRAPPER G, et al.Pressure-driven formation and stabilization of superconductive chromium hydrides[J]. Scientific Reports, 2015, 5:17764. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4667211/ [43] ZHANG H D, JIN X L, LV Y Z, et al.Investigation of stable germane structures under high-pressure[J]. Physical Chemistry Chemical Physics, 2015, 17(41):27630-27635. doi: 10.1039/C5CP03807C [44] LI X F, LIU H Y, PENG F.Crystal structures and superconductivity of technetium hydrides under pressure[J]. Physical Chemistry Chemical Physics, 2016, 18(41):28791-28796. doi: 10.1039/C6CP05702K [45] LIU H Y, LI Y W, GAO G Y, et al.Crystal structure and superconductivity of PH3 at high pressures[J]. The Journal of Physical Chemistry C, 2016, 120(6):3458-3461. doi: 10.1021/acs.jpcc.5b12009 [46] PÉPIN C M, LOUBEYRE P.Layered structure and re-entrant disproportionation observed in crystalline BeH2 under pressure[J]. Physical Review B, 2016, 93(22):224104. doi: 10.1103/PhysRevB.93.224104 [47] SHAMP A, TERPSTRA T, BI T, et al.Decomposition products of phosphine under pressure:PH2 stable and superconducting?[J]. Journal of the American Chemical Society, 2016, 138(6):1884-1892. doi: 10.1021/jacs.5b10180 [48] PICKARD C J, MARTINEZ-CANALES M, NEEDS R J.Decomposition and terapascal phases of water ice[J]. Physical Review Letters, 2013, 110(24):245701. doi: 10.1103/PhysRevLett.110.245701 [49] FLORES-LIVAS J A, SANNA A, GROSS E K U.High temperature superconductivity in sulfur and selenium hydrides at high pressure[J]. The European Physical Journal B, 2016, 89(3):63. doi: 10.1140/epjb/e2016-70020-0 [50] COLLINS M J, RATCLIFFE C I, RIPMEESTER J A.Deuteron and sulfur-33 NMR line-shape studies of the molecular motion in the liquid and solid phases of hydrogen sulfide and the solid Ⅱ phase of hydrogen selenide[J]. The Journal of Physical Chemistry, 1989, 93(21):7495-7502. doi: 10.1021/j100358a046 [51] LOEHLIN J H, MENNITT P G, WAUGH J S.Proton resonance study of molecular motion and phase behavior of solid H2S and H2Se[J]. The Journal of Chemical Physics, 1966, 44(10):3912-3917. doi: 10.1063/1.1726551 [52] HAYNES W M.CRC handbook of chemistry and physics[M]. Boca Raton:CRC Press, 2014. [53] COCKCROFT J K, FITCH A N.The solid phases of deuterium sulphide by powder neutron diffraction[J]. Zeitschrift für Kristallographie-Crystalline Materials, 1990, 193(1):1-20. https://www.researchgate.net/publication/30413221_The_solid_phases_of_deuterium_sulphide_by_powder_neutron_diffraction [54] SHIMIZU H, NAKAMICHI Y, SASAKI S.Pressure-induced phase transition in solid hydrogen sulfide at 11 GPa[J]. The Journal of Chemical Physics, 1991, 95(3):2036-2040. doi: 10.1063/1.461002 [55] ENDO S, ICHIMIYA N, KOTO K, et al.X-ray-diffraction study of solid hydrogen sulfide under high pressure[J]. Physical Review B, 1994, 50(9):5865-5867. doi: 10.1103/PhysRevB.50.5865 [56] ENDO S, HONDA A, SASAKI S, et al.High-pressure phase of solid hydrogen sulfide[J]. Physical Review B, 1996, 54(2):R717-R719. doi: 10.1103/PhysRevB.54.R717 [57] SAKASHITA M, YAMAWAKI H, FUJIHISA H, et al.Pressure-induced molecular dissociation and metallization in hydrogen-bonded H2S solid[J]. Physical Review Letters, 1997, 79(6):1082-1085. doi: 10.1103/PhysRevLett.79.1082 [58] FUJIHISA H, YAMAWAKI H, SAKASHITA M, et al.Structures of H2S:phases Ⅰ' and Ⅳ under high pressure[J]. Physical Review B, 1998, 57(5):2651-2654. doi: 10.1103/PhysRevB.57.2651 [59] ENDO S, HONDA A, KOTO K, et al.Crystal structure of high-pressure phase-Ⅳ solid hydrogen sulfide[J]. Physical Review B, 1998, 57(10):5699-5703. doi: 10.1103/PhysRevB.57.5699 [60] ROUSSEAU R, BOERO M, BERNASCONI M, et al.Static structure and dynamical correlations in high pressure H2S[J]. Physical Review Letters, 1999, 83(11):2218-2221. doi: 10.1103/PhysRevLett.83.2218 [61] IKEDA T.Pressure-induced phase transition of hydrogen sulfide at low temperature:role of the hydrogen bond and short S-S contacts[J]. Physical Review B, 2001, 64(10):104103. doi: 10.1103/PhysRevB.64.104103 [62] WANG L C, TIAN F B, FENG W X, et al.Order-disorder phase transition and dissociation of hydrogen sulfide under high pressure:ab initio molecular dynamics study[J]. The Journal of Chemical Physics, 2010, 132(16):164506. doi: 10.1063/1.3392673 [63] ROUSSEAU R, BOERO M, BERNASCONI M, et al.Ab initio simulation of phase transitions and dissociation of H2S at high pressure[J]. Physical Review Letters, 2000, 85(6):1254-1257. doi: 10.1103/PhysRevLett.85.1254 [64] WANG Y C, LV J A, ZHU L, et al.Crystal structure prediction via particle-swarm optimization[J]. Physical Review B, 2010, 82(9):094116. doi: 10.1103/PhysRevB.82.094116 [65] WANG Y C, LV J, ZHU L, et al.CALYPSO:a method for crystal structure prediction[J]. Computer Physics Communications, 2012, 183(10):2063-2070. doi: 10.1016/j.cpc.2012.05.008 [66] STROBEL T A, GANESH P, SOMAYAZULU M, et al.Novel cooperative interactions and structural ordering in H2S-H2[J]. Physical Review Letters, 2011, 107(25):255503. doi: 10.1103/PhysRevLett.107.255503 [67] EINAGA M, SAKATA M, ISHIKAWA T, et al.Crystal structure of 200 K-superconducting phase of sulfur hydride system[J]. Nature Physics, 2016, 12(9):835-838. doi: 10.1038/nphys3760 [68] HUANG X L, WANG X, DUAN D F, et al. Direct meissner effect observation of superconductivity in compressed H2S[EB/OL]. [2017-11-01]. https://arxiv.org/abs/1610.02630. [69] GE Y F, ZHANG F, YAO Y G.First-principles demonstration of superconductivity at 280 K in hydrogen sulfide with low phosphorus substitution[J]. Physical Review B, 2016, 93(22):224513. doi: 10.1103/PhysRevB.93.224513 [70] FLORES-LIVAS J A, SANNA A, GROSS E K U.High temperature superconductivity in sulfur and selenium hydrides at high pressure[J]. The European Physical Journal B, 2016, 89(3):63. doi: 10.1140/epjb/e2016-70020-0 [71] ZUREK E, HOFFMANN R, ASHCROFT N W, et al.A little bit of lithium does a lot for hydrogen[J]. Proceedings of the National Academy of Sciences, 2009, 106(42):17640-17643. doi: 10.1073/pnas.0908262106 [72] WANG H, JOHN S T, TANAKA K, et al.Superconductive sodalite-like clathrate calcium hydride at high pressures[J]. Proceedings of the National Academy of Sciences, 2012, 109(17):6463-6466. doi: 10.1073/pnas.1118168109 [73] GAO G Y, OGANOV A R, BERGARA A, et al.Superconducting high pressure phase of germane[J]. Physical Review Letters, 2008, 101(10):107002. doi: 10.1103/PhysRevLett.101.107002 [74] TSE J S, YAO Y, TANAKA K.Novel superconductivity in metallic SnH4 under high pressure[J]. Physical Review Letters, 2007, 98(11):117004. doi: 10.1103/PhysRevLett.98.117004 [75] GAO G Y, OGANOV A R, LI P F, et al.High-pressure crystal structures and superconductivity of Stannane (SnH4)[J]. Proceedings of the National Academy of Sciences, 2010, 107(4):1317-1320. doi: 10.1073/pnas.0908342107 -






 下载:
下载: