Mixing and Explosion Process of Propane-Air at Lower Flammable Limit in Confined Vessel
-
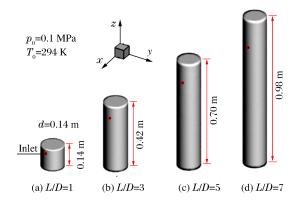
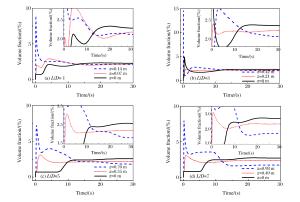
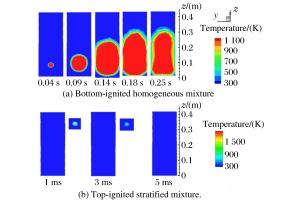
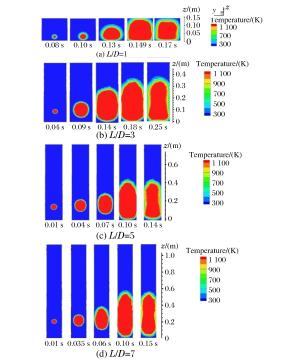
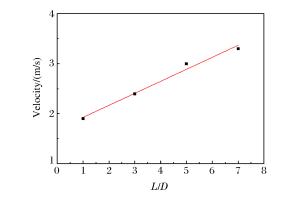
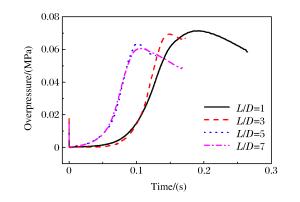
摘要: 丙烷是液化石油气的主要成分之一,爆炸下限的丙烷-空气浓度分布及其可燃性是液化石油气安全技术措施的基础。采用Fluent软件,建立三维数学模型,在长径比分别为1、3、5和7的容器内,模拟了爆炸下限的丙烷-空气混合过程和燃烧过程,分析了爆炸下限的丙烷-空气不均匀分布时对混合气体燃烧的影响。实验数据验证了该数值模型的合理性。在重力作用下丙烷-空气浓度分布不均匀,长径比增大,丙烷浓度梯度增大。浓度分布不均匀导致不同的点火位置对爆炸下限丙烷-空气燃烧有影响。容器长径比影响火焰传播,随着长径比增大,非均匀丙烷-空气混合气体超压峰值呈下降趋势,其超压峰值出现的时间变短。Abstract: Propane is one of the major constituents in petroleum gas, and its concentration distribution and flammability in the air is the basis of safety measures for petroleum gas.The influence of concentration distribution on the flammability of the propane-air mixture was analyzed using numerical simulation and experiment.The FLUENT, a computational fluid dynamics (CFD) software, was adopted to simulate the three-dimensional gas mixing process and the combustion process at a low flammability limit in non-adiabatic vessels with length-to-diameter ratios (L/D) of 1, 3, 5 and 7, respectively.The results show that the concentration distribution of the propane-air mixture is inhomogeneous by gravity, and the concentration distribution is the prime cause of the spark location affecting the combustion behavior of the propane-air at a low flammability limit.With the increase of the length-to-diameter ratio, the peak overpressure decreases.
-
Key words:
- mixing /
- explosion /
- propane-air /
- confined vessel /
- lower flammability limit
-
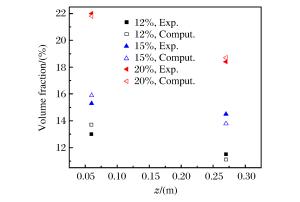
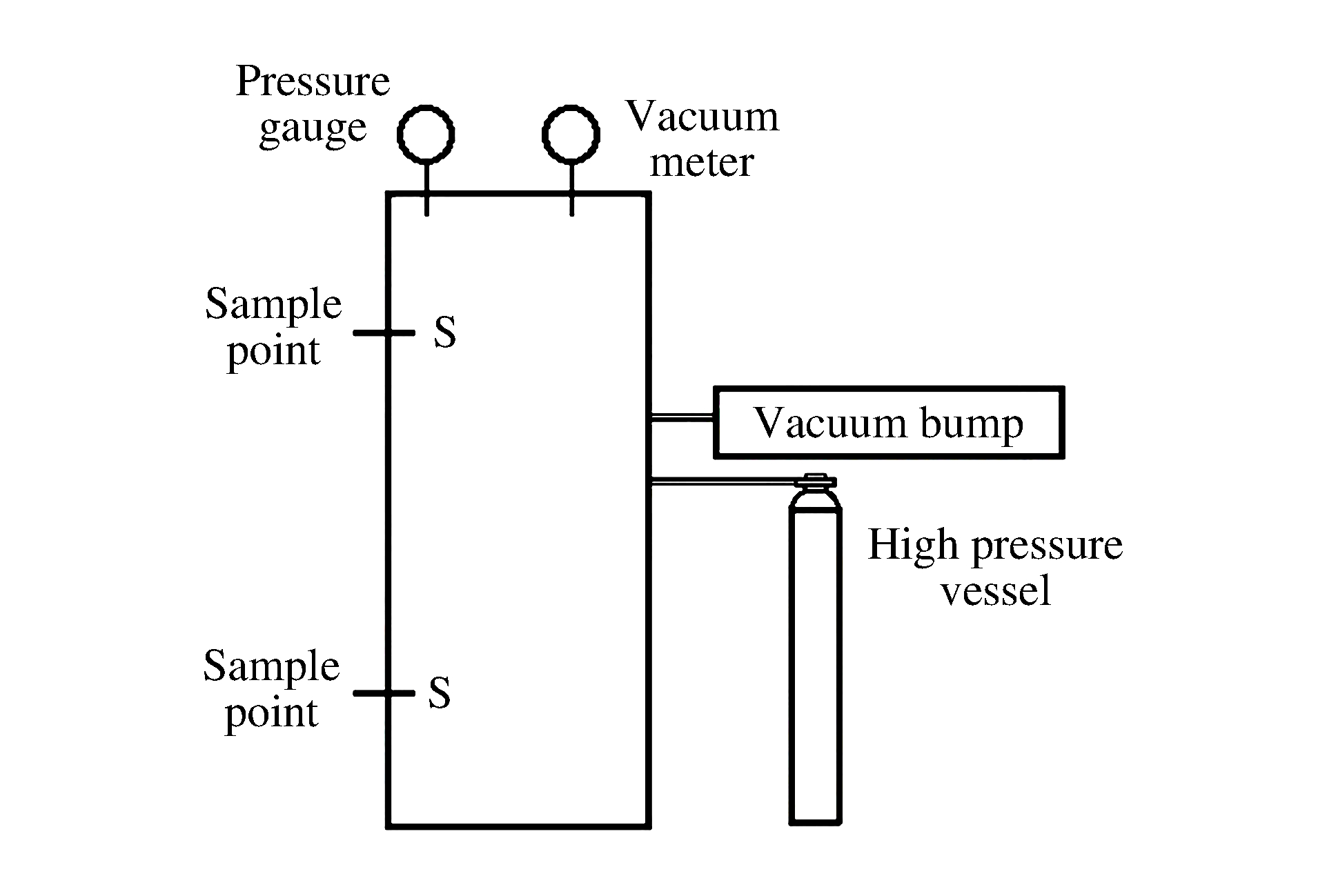
表 1 罐高1/5和4/5处测量的氧气浓度
Table 1. Measured volume fraction of O2 at 1/5 and 4/5 of vessel height
Height/
(m)12%(O2 volume fraction) 15%(O2 volume fraction) 20%(O2 volume fraction) 1 2 3 Average 1 2 3 Average 1 2 3 Average 0.06 13.1 12.8 13.1 13.0 15.6 15.5 15.2 15.4 22.2 21.8 22.1 22.0 0.27 11.7 11.4 11.3 11.5 14.5 14.6 14.3 14.5 18.2 18.9 18.3 18.5 表 2 Grid 1和Grid 2网格计算氧气浓度偏差对比
Table 2. Relative deviation of simulated concentration from two kinds of gird sizes(Grid 1, Grid 2)
Time/
(s)Concentration(Grid 1)/(%) Concentration(Grid 2)/(%) Concentration difference/(%) Relative deviation/(%) 5 14.600 14.320 0.280 1.90 30 14.510 14.320 0.190 1.31 40 14.508 14.319 0.189 1.30 表 3 Grid 3和Grid 4网格计算超压偏差
Table 3. Relative deviation of simulated peak overpressure from two kinds of gird sizes(Grid 3, Grid 4)
Item Grid 3 Grid 4 Difference Relative deviation/(%) Peak overpressure/(MPa) 0.071 5 0.071 3 0.000 2 0.3 Time of peak overpressure/(s) 0.187 0.195 0.008 4.2 -
[1] 邢志祥.火灾环境下液化气储罐热响应动力过程的研究[D].南京: 南京工业大学, 2004: 1-4. http://cdmd.cnki.com.cn/Article/CDMD-10291-2005128990.htmXING Z X.A study on the thermal response dynamic process of liquefied gas tanks subjected to fire environment[D].Nanjing: Nanjing University of Technology, 2004: 1-4. http://cdmd.cnki.com.cn/Article/CDMD-10291-2005128990.htm [2] 张增亮, 林柏泉, 李贤忠, 等.液化石油气爆炸抑制研究[J].燃烧科学与技术, 2013, 19(4):317-322. http://d.old.wanfangdata.com.cn/Periodical/rskxyjs201304006ZHANG Z L, LIN B Q, LI X Z, et al.Liquefied petroleum gas explosion suppression[J].Journal of Combustion Science and Technology, 2013, 19(4):317-322. http://d.old.wanfangdata.com.cn/Periodical/rskxyjs201304006 [3] SALZANO E, CAMMAROTA F, BENEDETTO A D, et al.Explosion behavior of hydrogen-methane/air mixtures[J].J Loss Prevent Proc, 2012, 25(3):443-447. doi: 10.1016/j.jlp.2011.11.010 [4] MIAO H Y, LU L, HUANG Z H.Flammability limits of hydrogen-enriched natural gas[J].Int J Hydrogen Energ, 2011, 36(11):6937-6947. doi: 10.1016/j.ijhydene.2011.02.126 [5] Fluent Inc.Fluent 6.3 user's guide [Z].Lebanon, NH: Fluent Inc, 2006: 1628-1641. [6] SCHMIDT D P, CORRADINI M L.Analytical prediction of the exit flow of cavitating orifices[J].Atomization Spray, 1997, 7(6):603-616. doi: 10.1615/AtomizSpr.v7.i6 [7] 王福军.计算流体动力学分析CFD软件原理与应用[M].北京:清华大学出版社, 2004:1-10.WANG F J.Computational fluid dynamics analysis—The principle and application of CFD software[M].Beijing:Tsinghua University Press, 2004:1-10. [8] COPPALLE A, VERVISCH P.The total emissivities of high-temperature flames[J].Combust Flame, 1983, 49(1):101-108. http://www.sciencedirect.com/science/article/pii/0010218083901542 [9] SMITH T F, SHEN Z F, FRIEDMAN J N.Evaluation of coecients for the weighted sum of gray gases model[J].J Heat Transfer, 1982, 104(4):602-608. doi: 10.1115/1.3245174 [10] CASHDOLLAR K L, ZLOCHOWER I A, GREEN G M, et al.Flammability of methane, propane, and hydrogen gases[J].J Loss Prevent Proc Ind, 2000, 13(3/4/5):327-340. http://www.sciencedirect.com/science/article/pii/S0950423099000376 -







 下载:
下载: