The Virial Coefficient of Argon for Pressure in the Megapascal Region
-
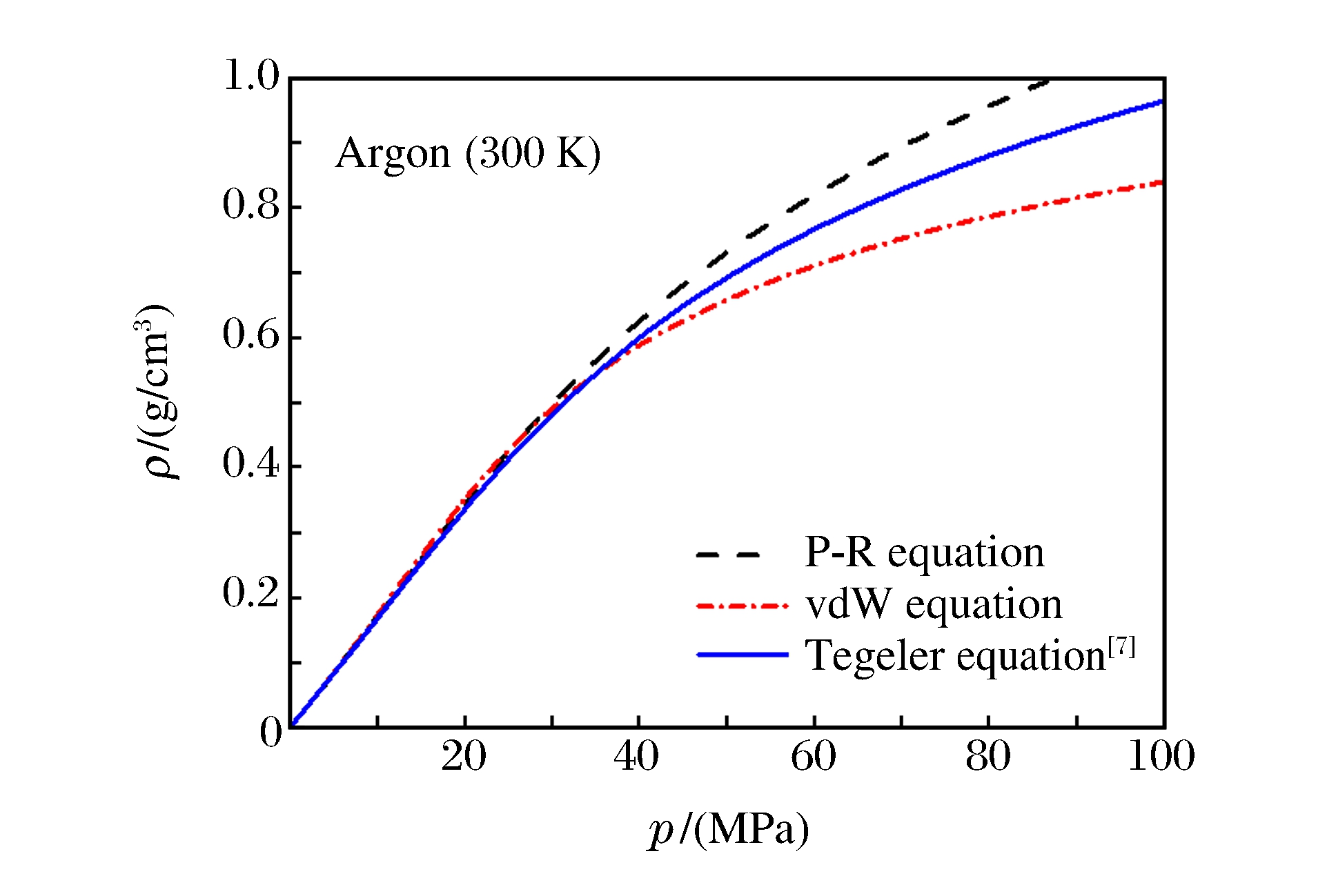
摘要: 设计了用于测量兆帕级压强范围内气体等温状态方程的实验装置,对300 K、0~60 MPa条件下氩气密度随压强的变化进行了精确测量。通过拟合实验数据,得到了氩气的第二和第三维里系数。由实验气体氩的维里系数,确定了其林纳德-琼斯势(L-J势)参数。采用该势参数对不同温度下氩气的状态方程进行了拓宽计算,并与其它实验和经验状态方程进行了比较分析,确定其适用范围。Abstract: Using a self-designed measuring device, the argon density has been measured at 300 K for pressure lower than 60 MPa.The second and third virial coefficients are obtained by fitting the experimental data, and the new parameters of Lennard-Jones potential are derived from the experimental virial coefficients.Furthermore, the temperature-dependent virial coefficients are calculated by these new potential parameters.The calculated densities from these virial equations are compared with other experiments and empirical equation of states for determining the application range of the present equation.
-
Key words:
- isothermal equation of state /
- argon /
- virial coefficient /
- density measurement
-
表 1 氩气密度测量数据
Table 1. Experimental data of argon density
T/(K) p/(MPa) ρ/(g/cm3) 298±0.5 5.15±0.01 0.086 2±0.000 9 299±0.5 10.52±0.01 0.180 5±0.001 8 299±0.5 20.47±0.01 0.348 1±0.003 4 298±0.5 30.60±0.01 0.480 9±0.002 4 299±0.5 40.35±0.01 0.592 3±0.003 0 300±0.5 55.00±0.01 0.720 3±0.003 6 表 2 由本实验势参数计算得到的不同温度下氩气的维里系数
Table 2. The temperature-dependent virial coefficients of argon computed by present potential parameters
T/(K) B/(cm3·mol-1) C/(cm6·mol-2) 200 -68.5 2 470 300 -23.2 2 014 400 -1.6 1 789 500 10.4 1 696 600 17.9 1 647 700 22.9 1 613 800 26.5 1 585 -
[1] Stevenson D J. Interiors of the giant planets[J]. Annu Rev Earth Planet Sci, 1982, 10: 257-295. doi: 10.1146/annurev.ea.10.050182.001353 [2] Christian R H, Yarger F L. Equation of state of gases by shock wave measurements. Ⅰ. Experimental method and the Hugoniot of argon[J]. J Chem Phys, 1955, 23(11): 2042-2044. doi: 10.1063/1.1740661 [3] Bespalov V E, Griaznov V K, Dremin A N, et al. Dynamic compression of non-ideal argon plasma[J]. Zh Eksp Teor Fiz, 1975, 69: 2059-2066. http://adsabs.harvard.edu/abs/1975ZhETF..69.2059B [4] Fortov V E, Leontev A A, Dremin A N, et al. Generation of nonideal plasma by strong shock wave[J]. Zh Eksp Teor Fiz, 1976, 71: 225. [5] van Thiel M, Shaner J, Salinas E. Compendium of shock wave data, UCRL-50108[R]. Livermore, USA: Lawrence Livermore National Laboratory, 1977. [6] Griaznov V K, Zhernokletov M V, Zubarev V N, et al. Thermodynamic properties of a nonideal argon or xenon plasma[J]. Zh Eksp Teor Fiz, 1980, 78: 573-585. http://adsabs.harvard.edu/abs/1980JETP...51..288G [7] Tegeler C, Span R, Wagner W. A new equation of state for argon covering the fluid region for temperatures from the melting line to 700 K at pressures up to 1 000 MPa[J]. J Phys Chem Ref Data, 1999, 28(3): 779-850. doi: 10.1063/1.556037 [8] Bird R B, Spotz E L, Hirschfelder J O. The third virial coefficient for non-polar gases[J]. J Chem Phys, 1950, 18(10): 1395-1402. doi: 10.1063/1.1747484 [9] 徐锡申, 张万箱.实用物态方程理论导引[M].北京: 科学出版社, 1986: 58-59.Xu X S, Zhang W X. Introduction to Theory of Applied Equations of State[M]. Beijing: Science Press, 1986: 58-59. (in Chinese) [10] Levine H B, McQuarrie D A. Second and third ordinary and dielectric virial coefficients for nonpolar axial molecules[J]. J Chem Phys, 1966, 44(9): 3500-3505. doi: 10.1063/1.1727256 [11] Michels A, Wijker H, Wijker H. Isotherms of argon between 0 ℃ and 150 ℃ and pressures up to 2 900 atmospheres[J]. Physica, 1949, 15(7): 627-633. doi: 10.1016/0031-8914(49)90119-6 [12] Vargaftik N B. Tables on the Thermophysical Properties of Liquids and Gases: In Normal and Dissociated States[M]. 2nd Ed. Washington D C, USA: Hemisphere Publishing Corporation, 1975: 543-570. -







 下载:
下载: