First-Principles Theoretical Study on the Structure Behaviors of NaPO3 under Compression
-
摘要: 探索PO6配位八面体的高压晶体化学行为是理解磷的高压化学性质、了解磷在下地幔中可能的赋存方式及磷的地球化学循环的重要基础。在0~80 GPa压力范围内,对MgSiO3等电子体的NaPO3开展第一性原理密度泛函理论研究,通过对其常压β相(P21/n)、透辉石相(C2/c)、钛铁矿相(R
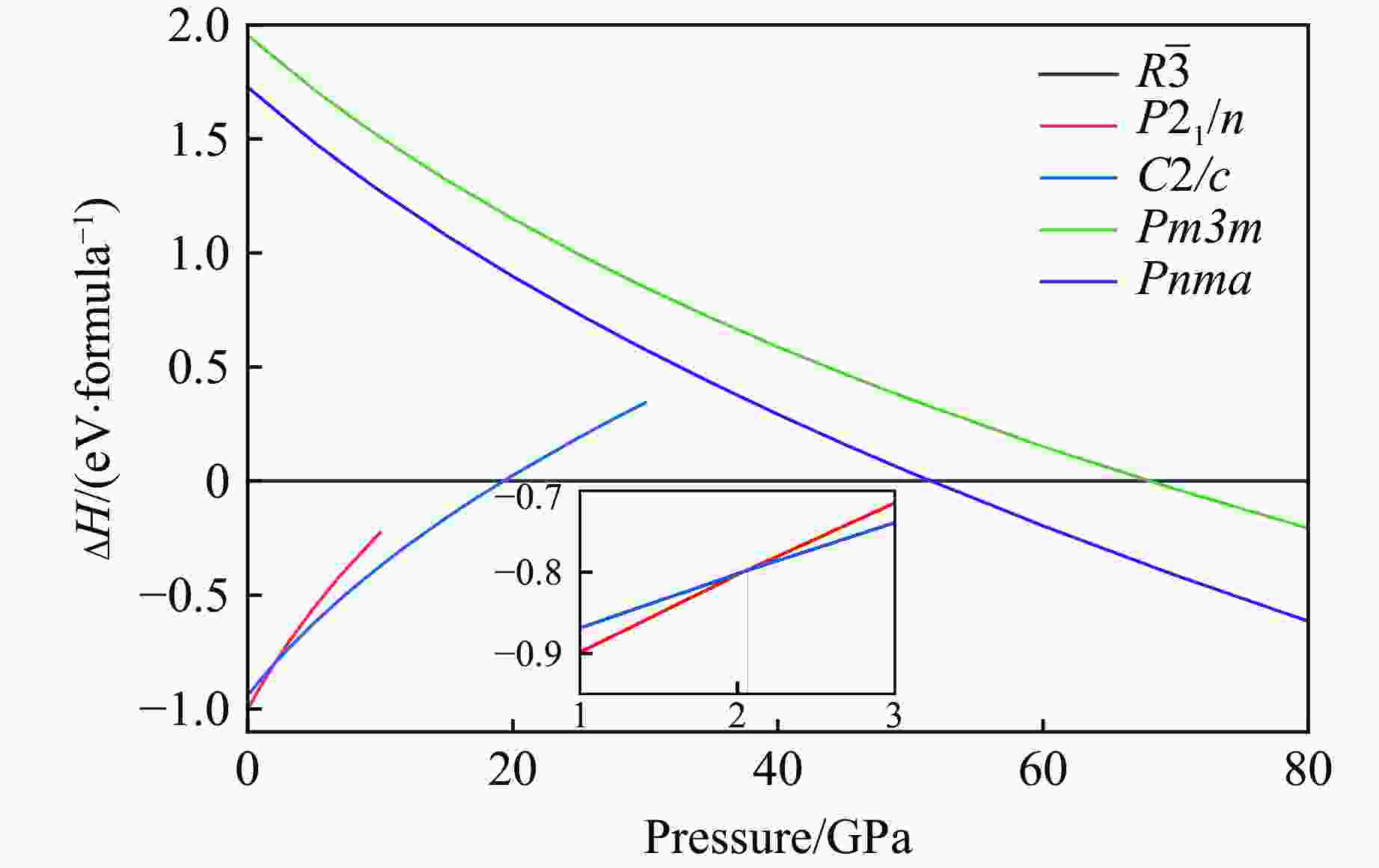
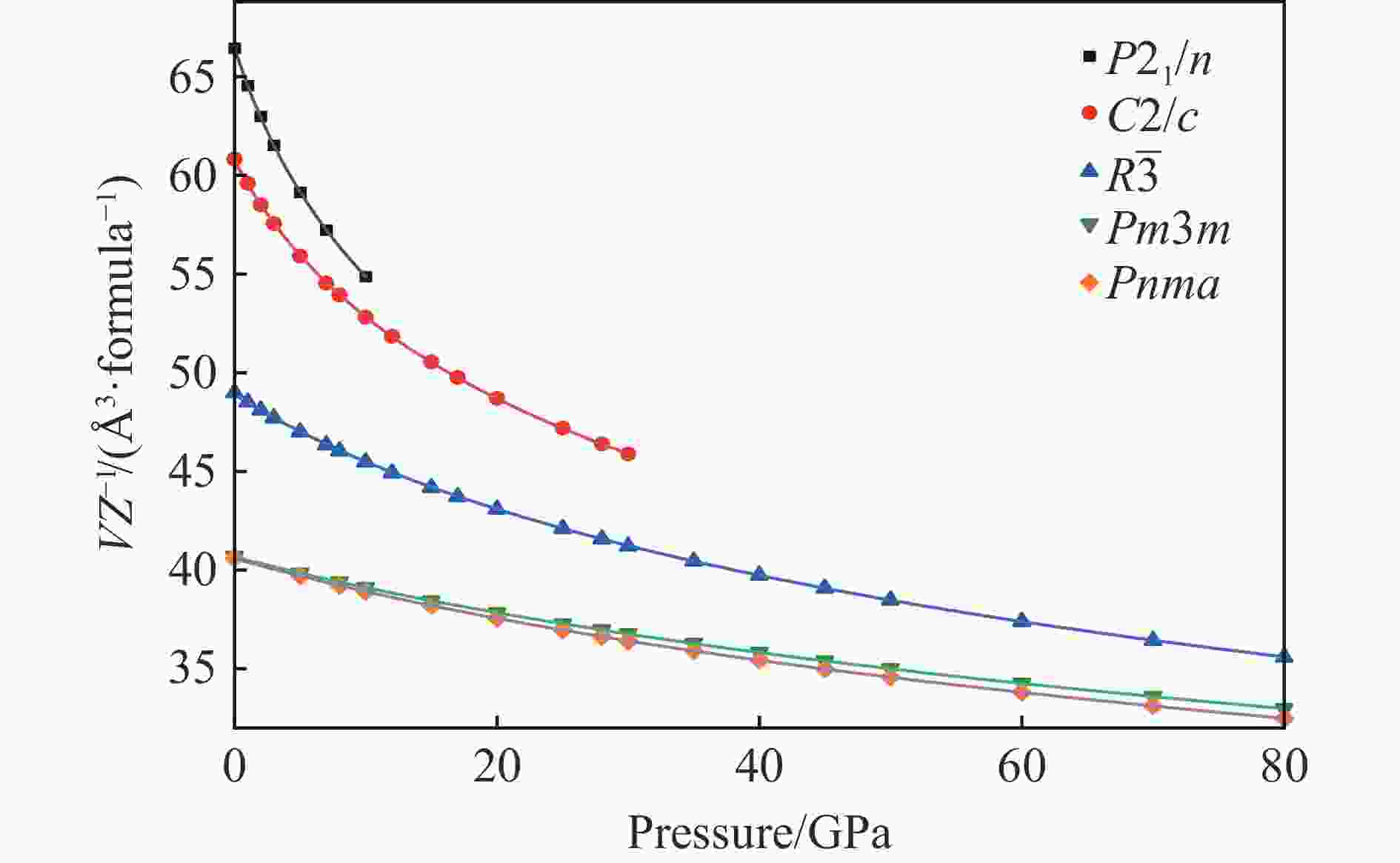
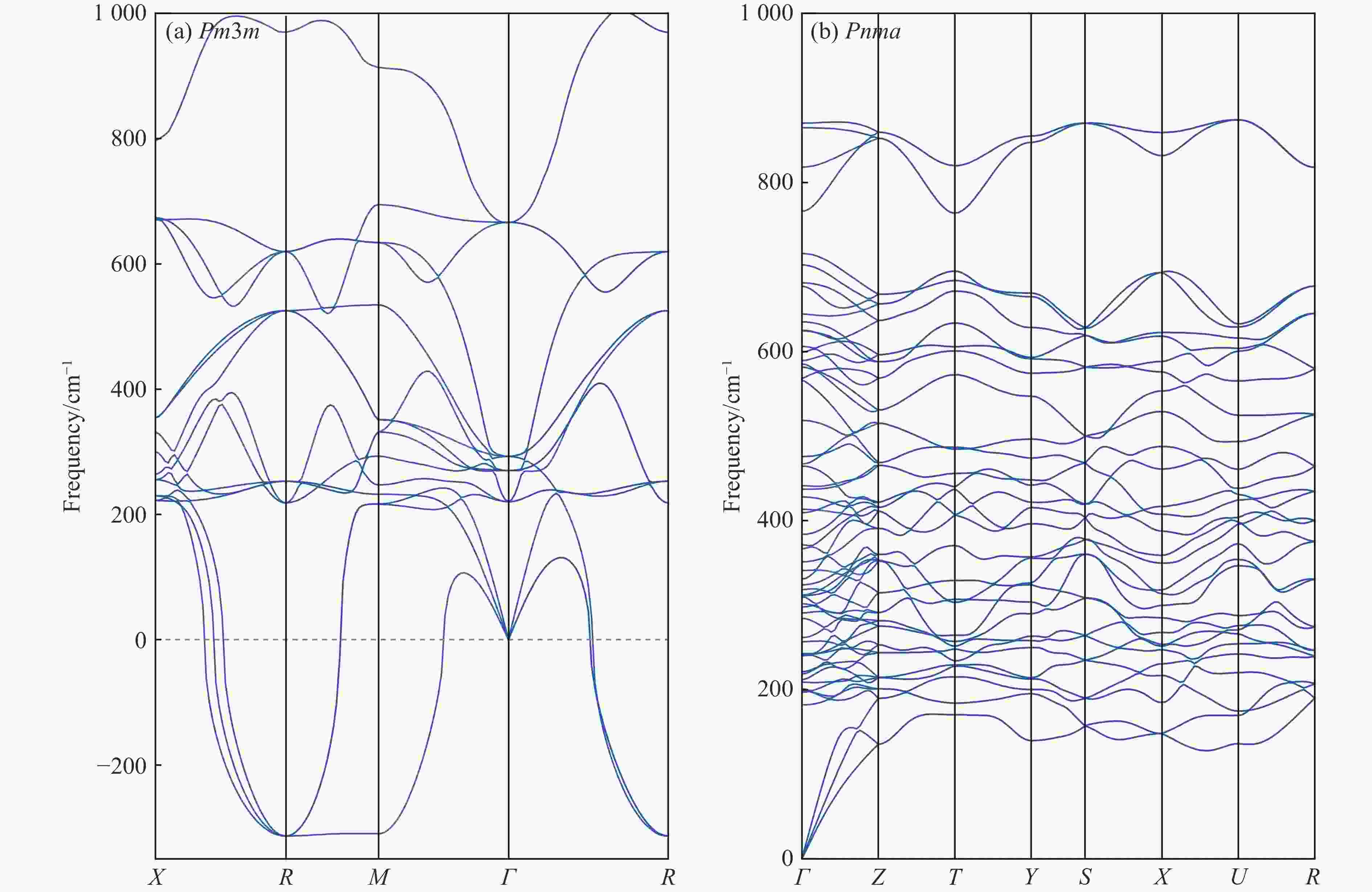
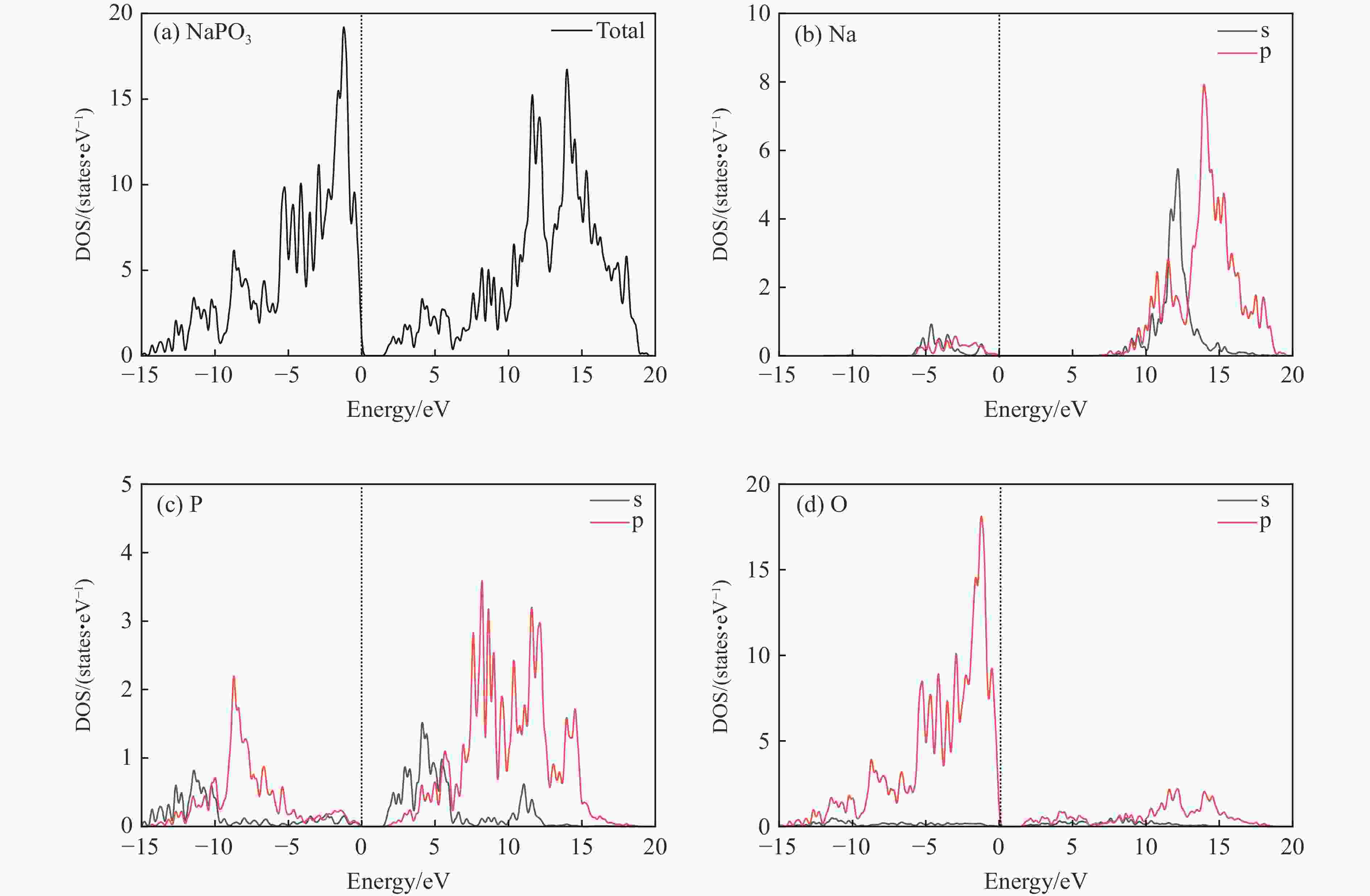
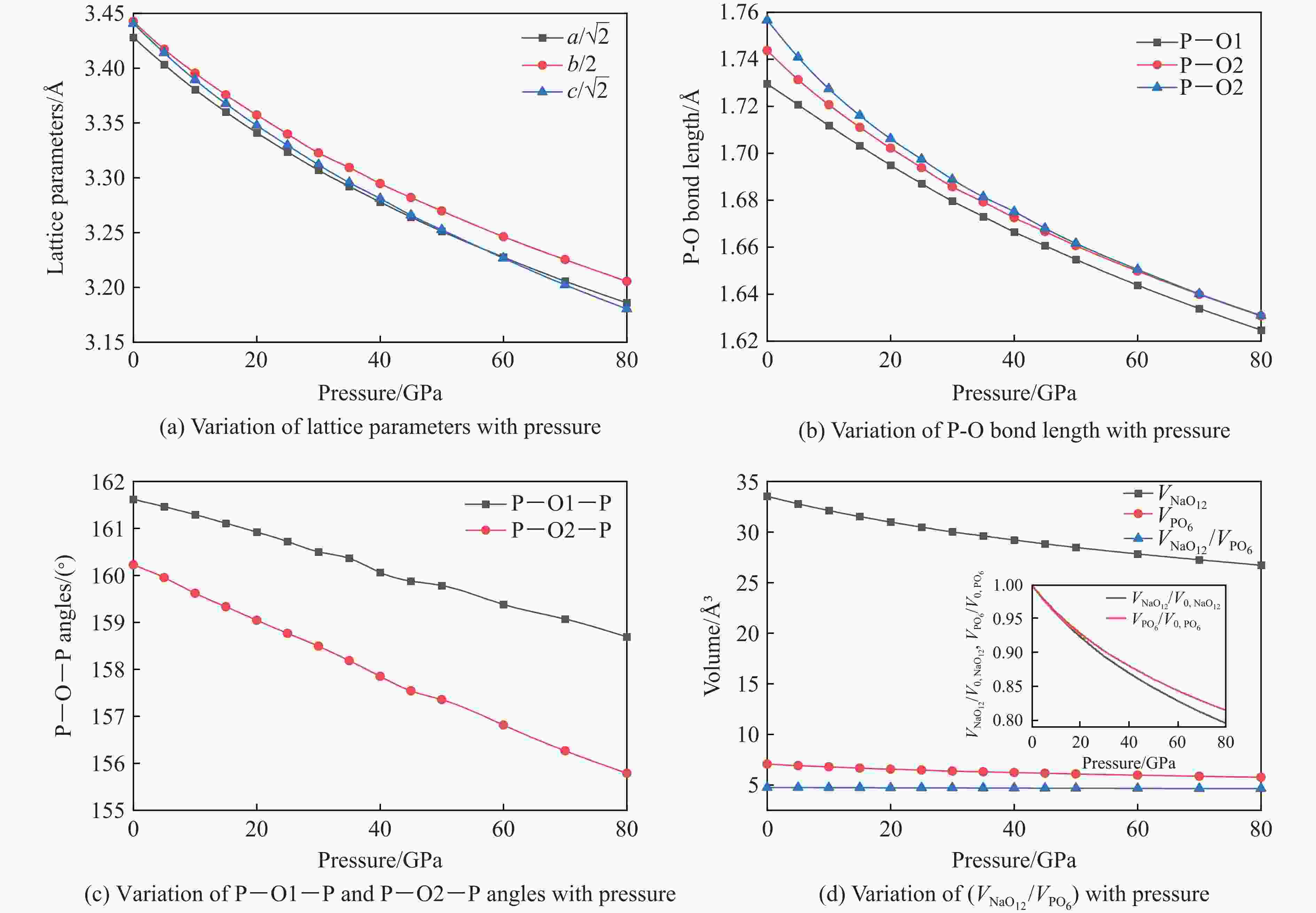
$ \overline 3 $ )、斜方钙钛矿相(Pnma)和立方钙钛矿相(Pm3m) 的几何优化和总能对比,获得了NaPO3的结构相变序列及相变压力:P21/n → C2/c (2 GPa) → R$ \overline 3 $ (20 GPa)→ Pnma(50 GPa),相变导致的体积变化分别为7.1%、11.5%和9.0%。Pm3m-NaPO3的声子色散曲线在R点和M点呈现出显著且相似的虚频,而Pnma-NaPO3在整个布里渊区均表现为实频,表明Pnma-NaPO3动力学稳定。Pnma-NaPO3的晶格常数、P―O键长、P―O―P键角、${{\mathrm{NaO}}_{12}} $ 和${{\mathrm{PO}}_6} $ 多面体体积比$V_{{\mathrm{NaO}}_{12}} $ /$V_{{\mathrm{PO}}_6} $ 与压力的关系表明,PO6八面体在计算的整个压力范围内都较规则,且NaO12多面体的压缩性比PO6八面体的压缩性更大。电子结构计算表明,在Pnma-NaPO3的PO6八面体中,P的3p和3s轨道与O的2p轨道强烈混合,P―O键表现出的强共价性对稳定其斜方钙钛矿结构发挥了关键作用。Abstract: Exploring the high-pressure crystal chemical behaviors of the PO6 coordinated octahedron is an important basis for understanding the high-pressure chemistry, the possible occurrence in the lower mantle, and the geochemical cycle of the phosphorus element. In this study, NaPO3, which is isoelectronic with the major component of the lower mantle MgSiO3, was studied with the first-principle density functional theory in the pressure range of 0–80 GPa. By ways of geometric optimization and total energy comparison of its ambient pressure β phase (P21/n), diopside phase (C2/c), ilmenite phase (R$ \overline 3 $ ), orthorhombic (Pnma) and cubic (Pm3m) perovskite phases, the structural phase transformation sequence and phase transformation pressures were obtained: P21/n→C2/c (2 GPa)→R$ \overline 3 $ (20 GPa)→Pnma (50 GPa), with the unit-cell volume changes of 7.1%, 11.5% and 9.0%, respectively. The phonon dispersion curves of Pm3m-NaPO3 show remarkable and similar imaginary frequencies at R and M points, while the orthorhombic perovskite structure shows real frequencies throughout the whole Brillouin zone reflecting its dynamic stability. The pressure dependence of lattice constants, P―O bond lengths, P―O―P bond angles and$V_{{\mathrm{NaO}}_{12}} $ /$V_{{\mathrm{PO}}_6} $ polyhedron volume ratio of Pnma-NaPO3 shows that the PO6 octahedron is regular in the whole calculated pressure range, and the compressibility of NaO12 polyhedron is greater than that of PO6 octahedron. The electronic structure calculation shows that the 3p and 3s orbitals of P are strongly mixed with 2p orbitals of O in the PO6 octahedron of Pnma-NaPO3, and the P―O bond exhibits strong covalency, which plays a key role in stabilizing the orthorhombic perovskite structure. -
表 1 0 GPa下NaPO3的5种结构在几何优化后的结构参数
Table 1. Structural parameters of five phases for NaPO3 at 0 GPa
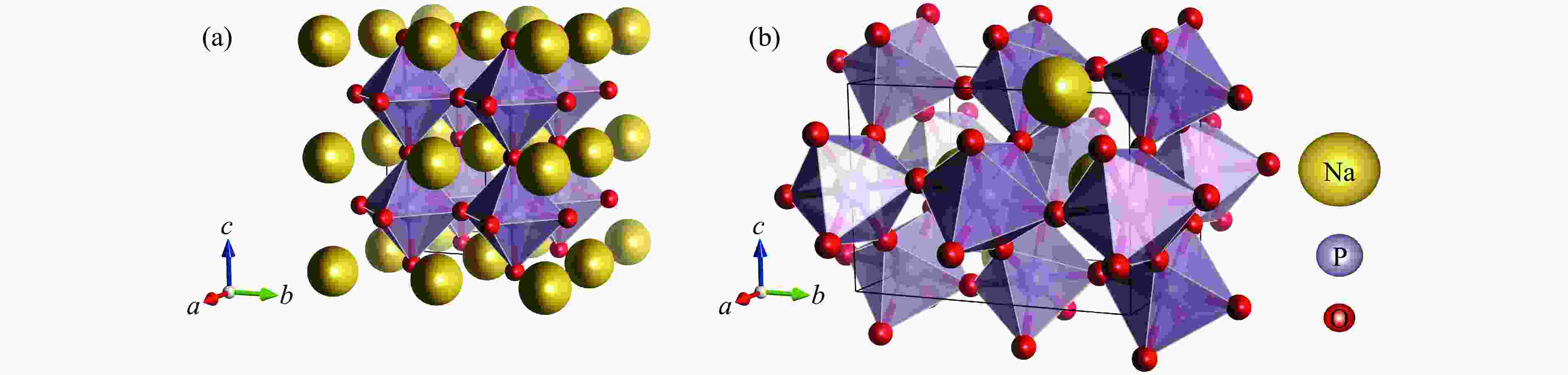
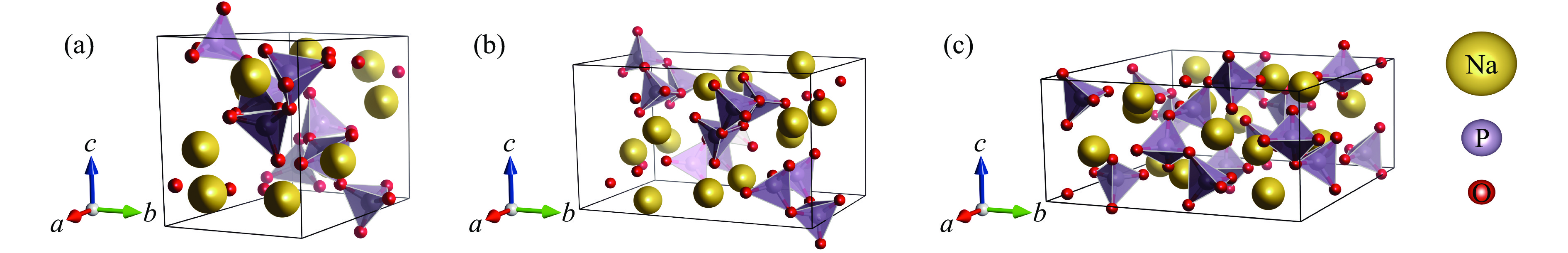
Phase VZ−1/(Å3·formula−1) a/Å b/Å c/Å β/(°) Atom Wyckoff site x y z P21/n 66.460 11.445 6.074 7.669 85.91 Na1 4e 0.1949 0.9249 0.6067 Na2 4e − 0.0001 0.3295 0.8093 P1 4e 0.1918 0.4206 0.4687 P2 4e 0.0963 0.1538 0.1823 O1 4e 0.1914 0.3465 0.6561 O2 4e 0.1559 0.1988 0.3661 O3 4e 0.1243 0.6207 0.4222 O4 4e 0.1723 0.9435 0.1085 O5 4e 0.1136 0.3513 0.0643 O6 4e 0.0236 0.9388 0.7763 C2/c 60.831 10.193 10.040 4.804 98.15 Na1 4e 0.5000 0.3967 0.2500 Na2 4e 0.5000 0.2112 0.7500 P 8f 0.2825 0.0880 0.2236 O1 8f 0.1338 0.0912 0.1854 O2 8f 0.3631 0.2083 0.3163 O3 8f 0.3358 0.0361 − 0.0639 R$ \overline 3 $ 48.990 4.618 15.915 Na 6c 0 0 0.3556 P 6c 0 0 0.1646 O 18f 0.3308 0.0527 0.2278 Pm3m 40.691 3.440 Na 1a 0 0 0 P 1b 0.5000 0.5000 0.5000 O 3c 0.5000 0.5000 0 Pnma 40.604 4.848 6.885 4.866 Na 4c 0.0240 0.2500 0.0042 P 4b 0.5000 0 0 O1 4c − 0.0053 0.2500 0.5567 O2 8d 0.2810 0.0285 0.2848 表 2 拟合得到的NaPO3的5种结构的状态方程参数
Table 2. Fitted parameters of equation of state for the five structures of NaPO3
Phase V0/Å3 K0/GPa $K_0' $ P21/n 531.84(32) 31.7(5) 5.16(13) C2/c 486.07(32) 47.8(5) 5.78(7) $R \overline 3 $ 293.35(12) 116.7(7) 4.81(3) Pnma 162.34(4) 215.7(1.1) 4.55(4) Pm3m 40.67(1) 235.6(1.2) 4.58(4) 表 3 0 GPa下Pnma-NaPO3的键长和键角
Table 3. Bond distances and bond angles of NaPO3 with orthorhombic perovskite structure (Pnma) at 0 GPa
Structure Type Bond distance/Å Bond angle/(º) PO6 P―O(1) [×2] 1.729 P―O(2) [×2] 1.744 P―O(2) [×2] 1.757 P―O1―P 161.62 P―O2―P [×2] 160.23 O1―P―O2 89.76 89.65 O2―P―O2 89.18 NaO12 Na―O(1) 2.181 Na―O(1) 2.301 Na―O(2) [×2] 2.184 Na―O(2) [×2] 2.390 Na―O(2) [×2] 2.396 Na―O(1) 2.583 Na―O(1) 2.693 Na―O(2) [×2] 2.800 -
[1] HUMINICKI D M C, HAWTHORNE F C. The crystal chemistry of the phosphate minerals [J]. Reviews in Mineralogy and Geochemistry, 2002, 48(1): 123–253. doi: 10.2138/rmg.2002.48.5 [2] BRUNET F, CHAZOT G. Partitioning of phosphorus between olivine, clinopyroxene and silicate glass in a spinel lherzolite xenolith from Yemen [J]. Chemical Geology, 2001, 176(1/2/3/4): 51–72. [3] WALTON C R, SHORTTLE O, JENNER F E, et al. Phosphorus mineral evolution and prebiotic chemistry: from minerals to microbes [J]. Earth-Science Reviews, 2021, 221: 103806. doi: 10.1016/j.earscirev.2021.103806 [4] MCDONOUGH W F, SUN S S. The composition of the Earth [J]. Chemical Geology, 1995, 120(3/4): 223–253. [5] BRUNET F, BONNEAU V, IRIFUNE T. Complete solid-solution between Na3Al2(PO4)3 and Mg3Al2(SiO4)3 garnets at high pressure [J]. American Mineralogist, 2006, 91(1): 211–215. doi: 10.2138/am.2006.2053 [6] WATSON E B, CHERNIAK D J, HOLYCROSS M E. Diffusion of phosphorus in olivine and molten basalt [J]. American Mineralogist, 2015, 100(10): 2053–2065. doi: 10.2138/am-2015-5416 [7] XING C M, WANG C Y, TAN W. Disequilibrium growth of olivine in mafic magmas revealed by phosphorus zoning patterns of olivine from mafic-ultramafic intrusions [J]. Earth and Planetary Science Letters, 2017, 479: 108–119. doi: 10.1016/j.jpgl.2017.09.005 [8] KONZETT J, FROST D J. The high P-T stability of hydroxyl-apatite in natural and simplified MORB—an experimental study to 15 GPa with implications for transport and storage of phosphorus and halogens in subduction zones [J]. Journal of Petrology, 2009, 50(11): 2043–2062. doi: 10.1093/petrology/egp068 [9] KONZETT J. From phosphates to silicates and back: an experimental study on the transport and storage of phosphorus in eclogites during uplift and exhumation [J]. American Mineralogist, 2016, 101(8): 1756–1768. doi: 10.2138/am-2016-5521 [10] YE K, CONG B L, YE D N. The possible subduction of continental material to depths greater than 200 km [J]. Nature, 2000, 407(6805): 734–736. doi: 10.1038/35037566 [11] PELLICER-PORRES J, SAITTA A M, POLIAN A, et al. Six-fold-coordinated phosphorus by oxygen in AlPO4 quartz homeotype under high pressure [J]. Nature Materials, 2007, 6(9): 698–702. doi: 10.1038/nmat1966 [12] BRUNET F, FLANK A M, ITIÉ J P, et al. Experimental evidence of sixfold oxygen coordination for phosphorus [J]. American Mineralogist, 2007, 92(7): 989–993. doi: 10.2138/am.2007.2570 [13] STEBBINS J F, KIM N, BRUNET F, et al. Confirmation of octahedrally coordinated phosphorus in AlPO4-containing stishovite by 31P NMR [J]. European Journal of Mineralogy, 2009, 21(4): 667–671. doi: 10.1127/0935-1221/2009/0021-1953 [14] PAKHOMOVA A, APRILIS G, BYKOV M, et al. Penta-and hexa-coordinated beryllium and phosphorus in high-pressure modifications of CaBe2P2O8 [J]. Nature Communications, 2019, 10(1): 2800. doi: 10.1038/s41467-019-10589-z [15] SALVADÓ M A, PERTIERRA P. Theoretical study of P2O5 polymorphs at high pressure: hexacoordinated phosphorus [J]. Inorganic Chemistry, 2008, 47(11): 4884–4890. doi: 10.1021/ic8001543 [16] LÓPEZ-SOLANO J, RODRÍGUEZ-HERNÁNDEZ P, MUÑOZ A, et al. Theoretical and experimental study of the structural stability of TbPO4 at high pressures [J]. Physical Review B, 2010, 81(14): 144126. doi: 10.1103/PhysRevB.81.144126 [17] LÓPEZ-MORENO S, ERRANDONEA D. Ab initio prediction of pressure-induced structural phase transitions of CrVO4-type orthophosphates [J]. Physical Review B, 2012, 86(10): 104112. doi: 10.1103/PhysRevB.86.104112 [18] JOST K H. Die struktur des Kurrol’schen Na-salzes (NaPO3) x typ A [J]. Acta Crystallographica, 1961, 14(8): 844–847. doi: 10.1107/S0365110X6100245X [19] JOST K H. Die struktur des Kurrol’schen Na-salzes (NaPO3) x, typ B [J]. Acta Crystallographica, 1963, 16(7): 640–642. doi: 10.1107/S0365110X63001687 [20] ONDIK H M. The structure of anhydrous sodium trimetaphosphate Na3P3O9, and the monohydrate, Na3P3O9·H2O [J]. Acta Crystallographica, 1965, 18(2): 226–232. doi: 10.1107/S0365110X65000518 [21] IMMIRZI A, PORZIO W. A new form of sodium Kurrol salt studied by the Rietveld method from X-ray diffraction data [J]. Acta Crystallographica Section B, 1982, 38(11): 2788–2792. doi: 10.1107/S0567740882009960 [22] THILO E. The structural chemistry of condensed inorganic phosphates [J]. Angewandte Chemie International Edition, 1965, 4(12): 1061–1071. doi: 10.1002/anie.196510611 [23] DURIF A. Crystal chemistry of condensed phosphates [M]. New York: Springer, 1995. [24] KORNBERG A, RAO N N, AULT-RICHÉ D. Inorganic polyphosphate: a molecule of many functions [J]. Annual Review of Biochemistry, 1999, 68: 89–125. doi: 10.1146/annurev.biochem.68.1.89 [25] AKBARI A, WANG Z J, HE P S, et al. Unrevealed roles of polyphosphate-accumulating microorganisms [J]. Microbial Biotechnology, 2021, 14(1): 82–87. doi: 10.1111/1751-7915.13730 [26] GASPARIK T. Phase diagrams for geoscientists: an atlas of the Earth’s interior [M]. 2nd ed. New York: Springer, 2014. [27] ANGEL R J, CHOPELAS A, ROSS N L. Stability of high-density clinoenstatite at upper-mantle pressures [J]. Nature, 1992, 358(6384): 322–324. doi: 10.1038/358322a0 [28] HORIUCHI H, HIRANO M, ITO E, et al. MgSiO3 (ilmenite-type): single crystal X-ray diffraction study [J]. American Mineralogist, 1982, 67(7/8): 788–793. [29] HORIUCHI H, ITO E, WEIDNER D J. Perovskite-type MgSiO3: single-crystal X-ray diffraction study [J]. American Mineralogist, 1987, 72(3/4): 357–360. [30] ANGEL R J, HUGH-JONES D A. Equations of state and thermodynamic properties of enstatite pyroxenes [J]. Journal of Geophysical Research: Solid Earth, 1994, 99(B10): 19777–19783. doi: 10.1029/94JB01750 [31] SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides [J]. Acta Crystallographica Section A, 1976, 32(5): 751–767. doi: 10.1107/S0567739476001551 [32] WOODWARD P M. Octahedral tilting in perovskites. Ⅰ. geometrical considerations [J]. Acta Crystallographica Section B, 1997, 53(1): 32–43. doi: 10.1107/S0108768196010713 [33] GLAZER A M. The classification of tilted octahedra in perovskites [J]. Acta Crystallographica Section B, 1972, 28(11): 3384–3392. doi: 10.1107/S0567740872007976 [34] ALEKSANDROV K S. The sequences of structural phase transitions in perovskites [J]. Ferroelectrics, 1976, 14(1): 801–805. doi: 10.1080/00150197608237799 [35] WOODWARD P M. Octahedral tilting in perovskites. Ⅱ. structure stabilizing forces [J]. Acta Crystallographica Section B, 1997, 53(1): 44–66. doi: 10.1107/S0108768196012050 [36] STIXRUDE L, COHEN R E, YU R C, et al. Prediction of phase transition in CaSiO3 perovskite and implications for lower mantle structure [J]. American Mineralogist, 1996, 81(9/10): 1293–1296. [37] BROWN I D, ALTERMATT D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database [J]. Acta Crystallographica Section B, 1985, 41(4): 244–247. doi: 10.1107/S0108768185002063 [38] MIZOGUCHI H, ENG H W, WOODWARD P M. Probing the electronic structures of ternary perovskite and pyrochlore oxides containing Sn4+ or Sb5+ [J]. Inorganic Chemistry, 2004, 43(5): 1667–1680. doi: 10.1021/ic034551c [39] MIZOGUCHI H, WOODWARD P M, BYEON S H, et al. Polymorphism in NaSbO3: structure and bonding in metal oxides [J]. Journal of the American Chemical Society, 2004, 126(10): 3175–3184. doi: 10.1021/ja038365h [40] SASAKI S, PREWITT C T, BASS J D, et al. Orthorhombic perovskite CaTiO3 and CdTiO3: structure and space group [J]. Acta Crystallographica Section C, 1987, 43(9): 1668–1674. [41] MAREZIO M, REMEIKA J P, DERNIER P D. The crystal chemistry of the rare earth orthoferrites [J]. Acta Crystallographica Section B, 1970, 26(12): 2008–2022. doi: 10.1107/S0567740870005319 [42] ZHOU J S, GOODENOUGH J B. Universal octahedral-site distortion in orthorhombic perovskite oxides [J]. Physical Review Letters, 2005, 94(6): 065501. doi: 10.1103/PhysRevLett.94.065501 [43] MARTÍNEZ-LOPE M J, ALONSO J A, RETUERTO M, et al. Evolution of the crystal structure of RVO3 (R=La, Ce, Pr, Nd, Tb, Ho, Er, Tm, Yb, Lu, Y) perovskites from neutron powder diffraction data [J]. Inorganic Chemistry, 2008, 47(7): 2634–2640. doi: 10.1021/ic701969q [44] THOMAS N W. A new global parameterization of perovskite structures [J]. Acta Crystallographica Section B, 1998, 54(5): 585–599. doi: 10.1107/S0108768198001979 [45] AVDEEV M, CASPI E N, YAKOVLEV S. On the polyhedral volume ratios VA/VB in perovskites ABX3 [J]. Acta Crystallographica Section B, 2007, 63(3): 363–372. doi: 10.1107/S0108768107001140 -














 下载:
下载: