Influence Mechanism of Different Magnetic Wires on Hydrogen Explosion
-
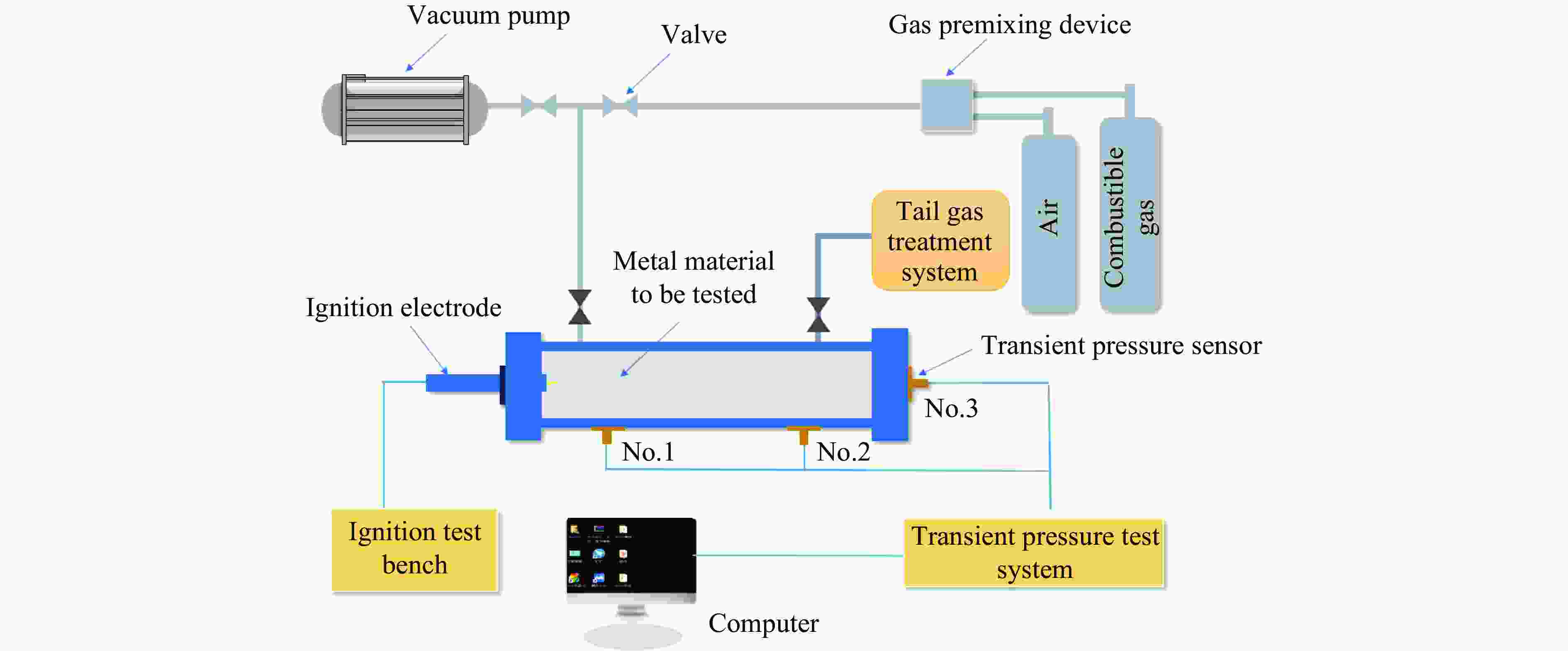
摘要: 为探索氢气爆炸防治新技术,开发新型阻隔防爆材料,开展了抗磁性铝丝和铁磁性镍丝对预混氢气-空气爆炸压力影响实验,利用CHEMKIN-PRO软件对氢气爆炸过程中的反应路径和温度敏感性变化进行模拟。实验结果表明,两种金属丝对氢气-空气混合气体爆炸具有双重作用:当混合气体中氢气的体积分数低于20%时,金属丝材料抑制氢气爆炸,且材料填充量越大,抑制作用越强;当混合气体中氢气的体积分数高于25%时,两种金属丝促进氢气爆炸,且填充量越大,促进作用越强。在促进爆炸阶段,镍丝的促进效果弱于铝丝;在抑制爆炸阶段,镍丝的抑爆效果优于铝丝。模拟结果表明,R2对氢气的生成速率影响最大,R1对氢气及爆炸过程中的温度影响最大,影响温度敏感性变化的主要基元反应对爆炸均具有促进作用。通过实验和数值模拟综合分析,揭示了不同磁性金属丝对氢气爆炸的影响机理,可为氢气爆炸防治和开发新型阻隔防爆材料提供理论指导。Abstract: For exploring the new technologies of hydrogen explosion prevention and the development of new barrier and explosion-proof materials, the effects of anti-magnetic aluminum wire and ferromagnetic nickel wire on premixed hydrogen-air explosion pressure were carried out. The CHEMKIN-PRO software was used to simulate the reaction path and temperature sensitivity changes during hydrogen explosion. The experimental results show that the two metal wires have a dual effect on the explosion of hydrogen-air mixture. When the volume fraction of hydrogen in the mixture is less than 20%, the metal wire material inhibits hydrogen explosion, and the larger the filling amount of the material, the stronger the inhibition. When the volume fraction of hydrogen in the mixed gas is higher than 25%, the two metal wires promote hydrogen explosion, and the larger the filling amount, the stronger the promotion. In the stage of promoting explosion, the effect of nickel wire is weaker than that of aluminum wire, in the explosion suppression stage, the explosion suppression effect of nickel wire is better than that of aluminum wire. The inhibition or promotion effect of metal materials on gas explosion is determined by the concentration and properties of gas and the filling amount of materials. Changing the filling amount of materials will lead to changes in the performance of inhibiting/promoting hydrogen explosion. The simulation results show that •H, •O, and •OH are the key free radicals in the process of hydrogen explosion, and the change of reaction rate and sensitivity directly determine the explosion intensity. Among the main elementary reactions that affect hydrogen explosion, R2 has the greatest impact on the formation rate of hydrogen, and R1 has the greatest impact on hydrogen and temperature during explosion. The main elementary reactions that affect the change of temperature sensitivity have a promoting effect on explosion. The influence mechanism of different magnetic wires on hydrogen explosion was revealed by experiment and numerical simulation.
-
Key words:
- hydrogen explosion /
- magnetism /
- temperature sensitivity /
- filling amount /
- free radicals /
- explosion-proof materials
-
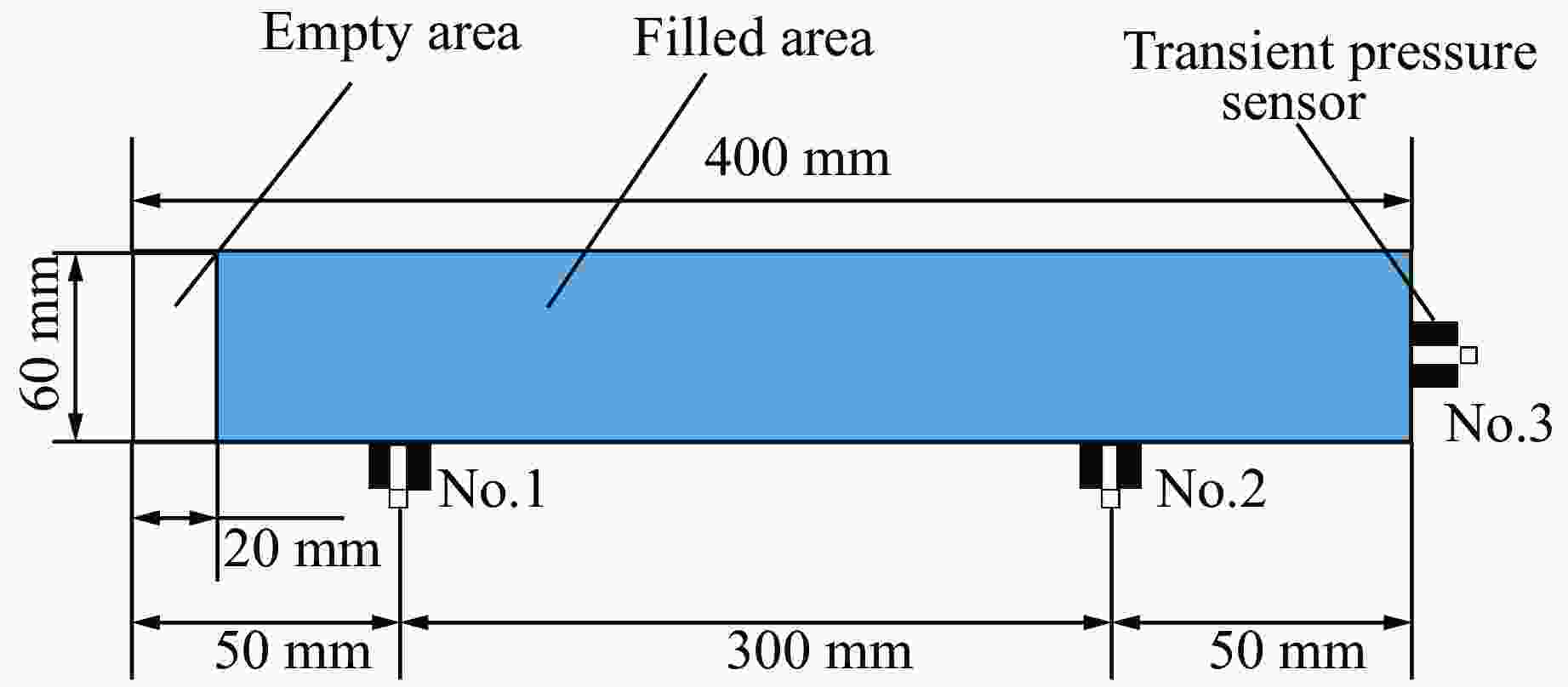
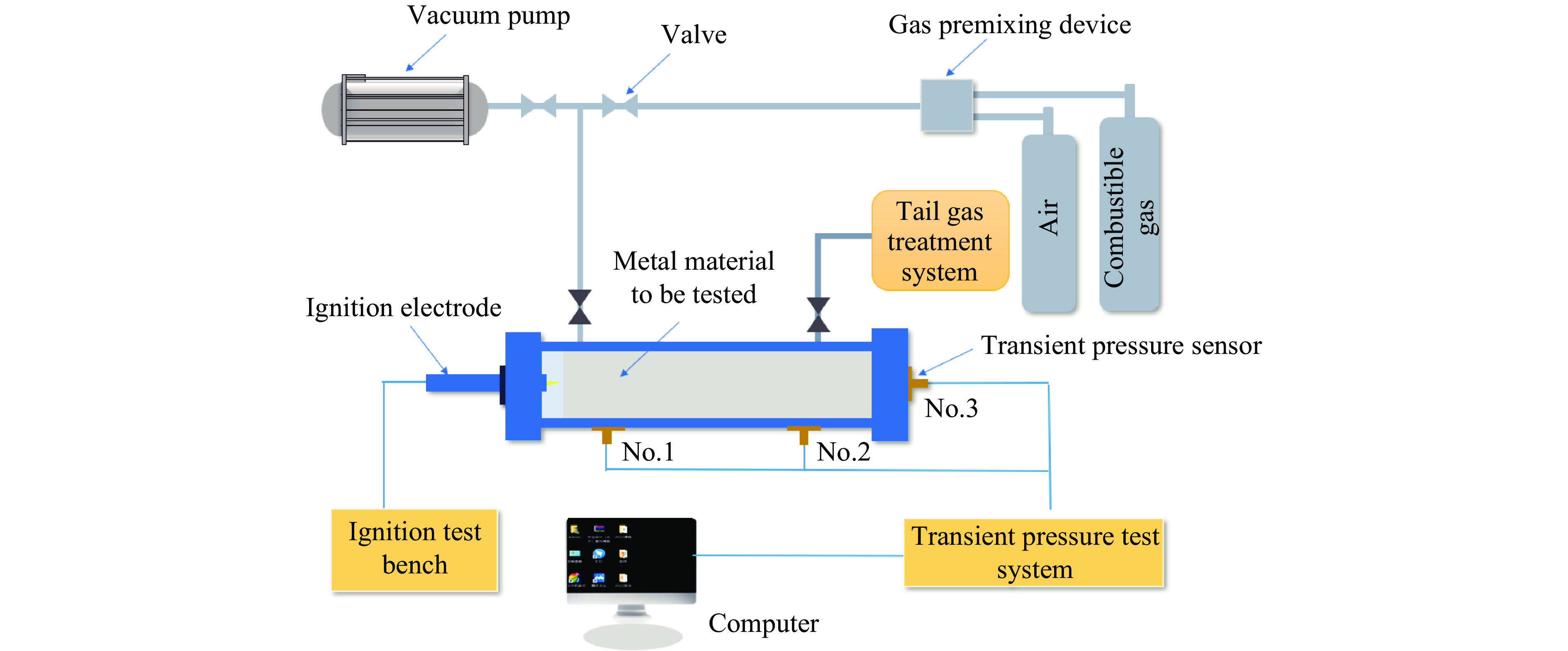
表 1 实验参数与工况
Table 1. Experimental parameters and working conditions
Exp. No. $\varphi $/% Filling material Filling surface area/cm2 1 15 Empty 0 2 15 Aluminium 2054, 2465, 2876 3 15 Nickel 2054, 2465, 2876 4 20 Empty 0 5 20 Aluminium 2054, 2465, 2876 6 20 Nickel 2054, 2465, 2876 7 25 Empty 0 8 25 Aluminium 2054, 2465, 2876 9 25 Nickel 2054, 2465, 2876 表 2 不同工况下空白组压力数据
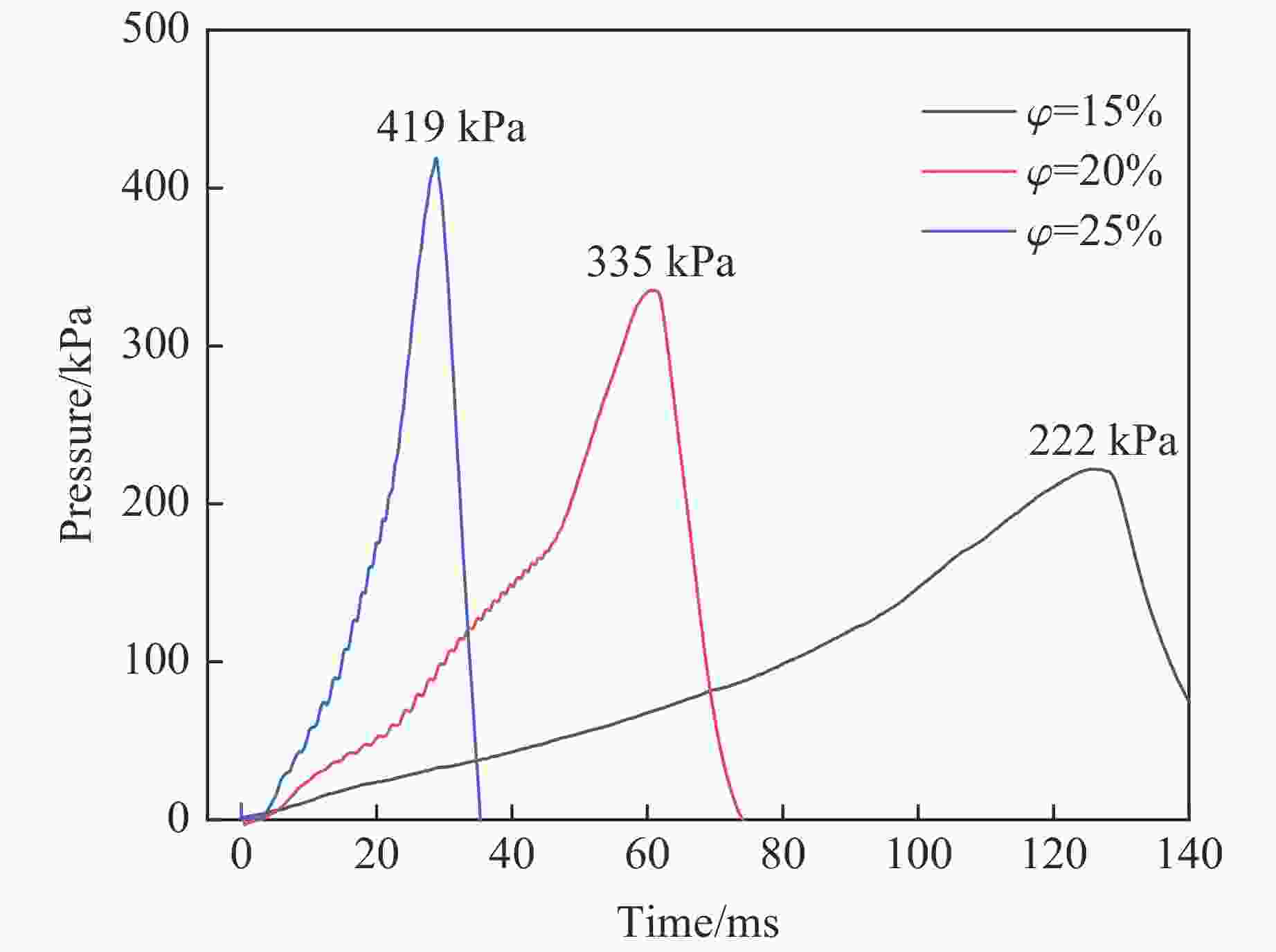
Table 2. Pressure data of blank group under different working conditions
$\varphi $/% pmax/kPa Δt/ms 15 222 125 20 335 60 25 419 29 表 3 不同工况下铝丝组的压力数据
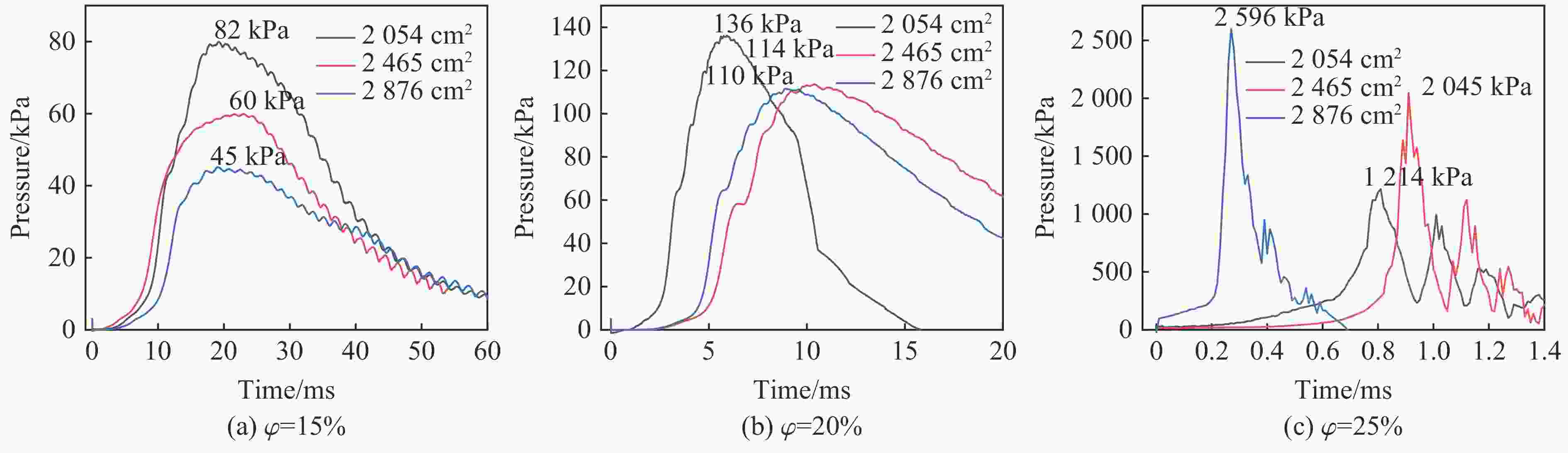
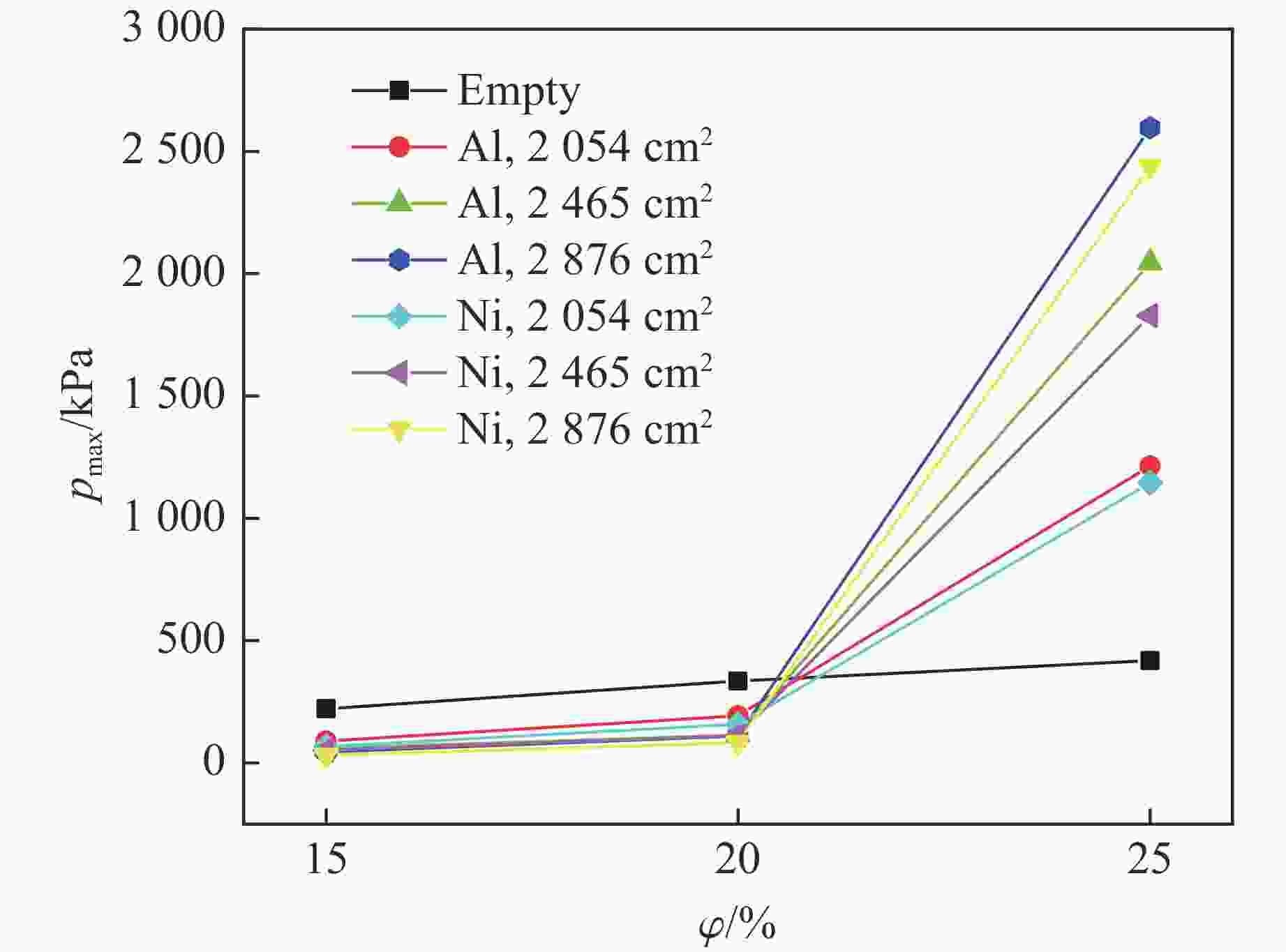
Table 3. Pressure data of aluminum wire group under different working conditions
Filling surface area/cm2 $\varphi $/% pmax/kPa Δt/ms 2054 15 82 19.20 20 136 18.86 25 1214 0.81 2465 15 60 22.98 20 114 10.38 25 2045 0.91 2876 15 45 19.08 20 110 8.91 25 2596 0.27 表 4 不同工况下镍丝组的压力数据
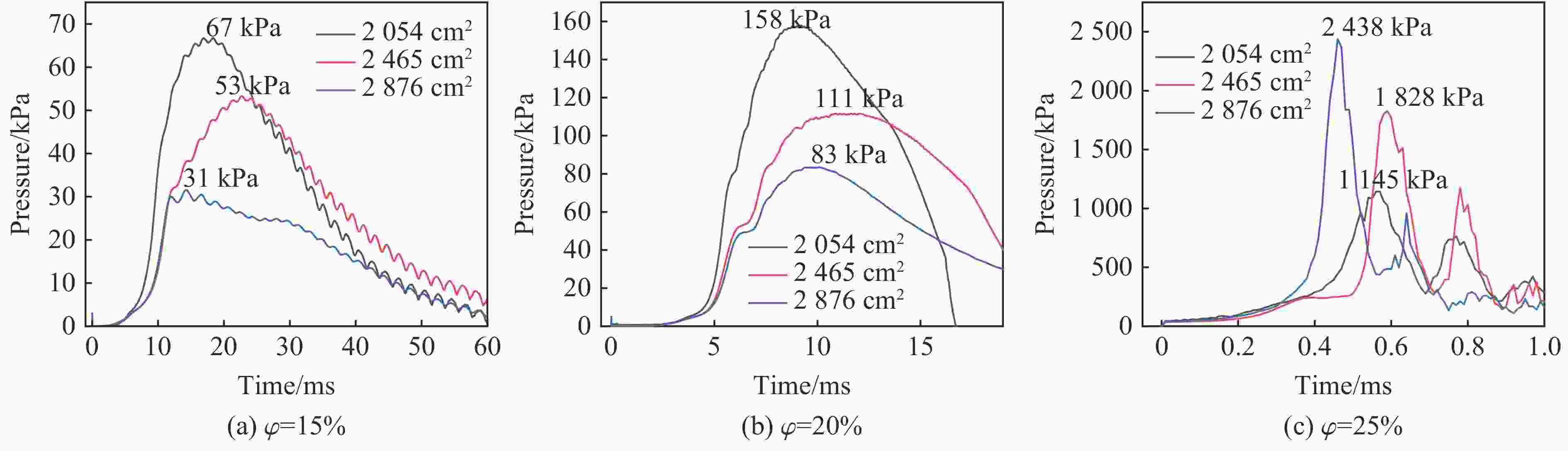
Table 4. Pressure data of nickel wire group under different working conditions
Filling surface area/cm2 $\varphi $/% pmax/kPa Δt/ms 2054 15 67 18.39 20 158 9.21 25 1145 0.57 2465 15 53 22.70 20 111 12.02 25 1828 0.59 2876 15 31 14.26 20 83 10.11 25 2438 0.46 表 5 氢气爆炸过程中的关键基元反应
Table 5. Key elementary reactions during hydrogen explosion
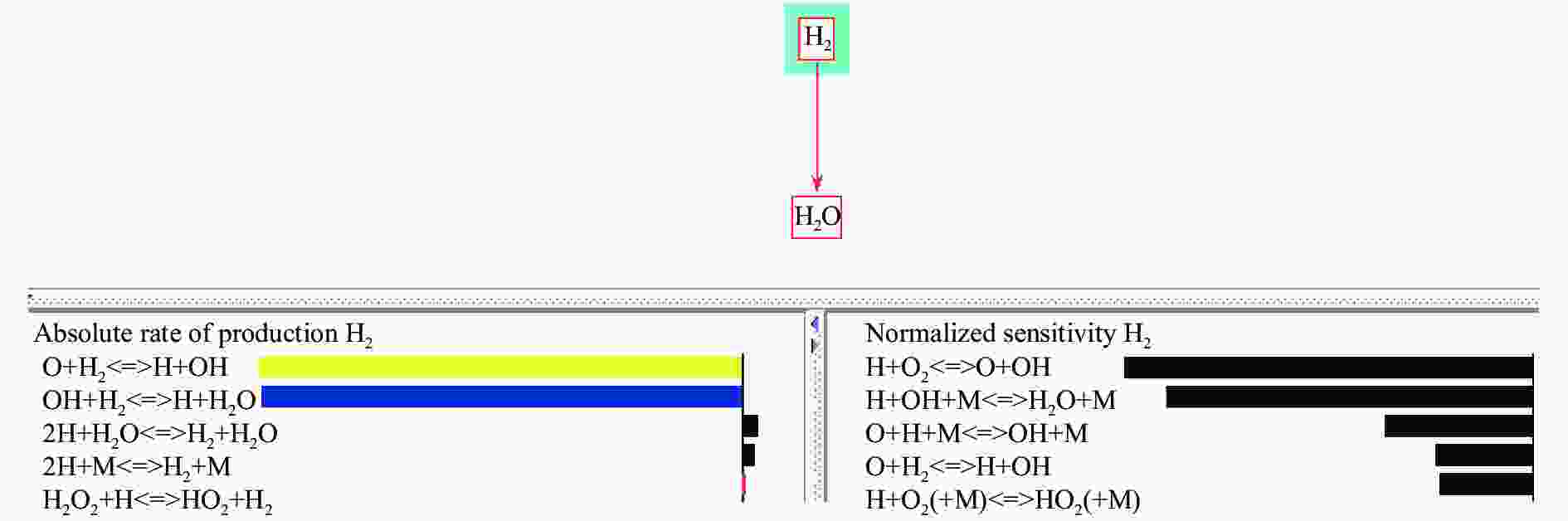
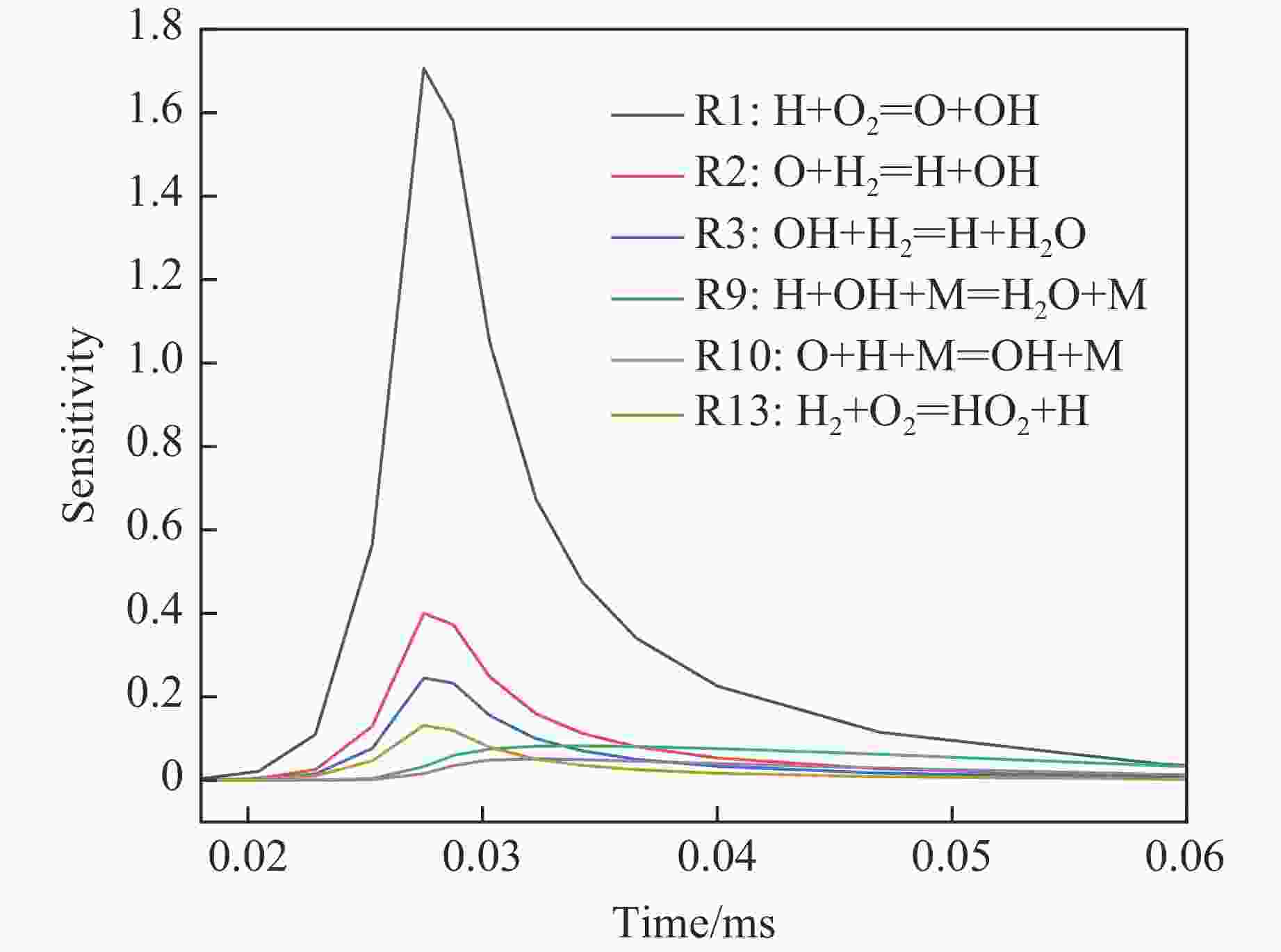
Reaction order Elementary reaction R1 H+O2=O+OH R2 O+H2=H+OH R3 OH+H2=H+H2O R9 H+OH=H2O R10 O+H=OH -
[1] ZHOU S Y, LUO Z M, WANG T, et al. Research progress on the self-ignition of high-pressure hydrogen discharge: a review [J].International Journal of Hydrogen Energy, 2022, 47(15): 9460–9476. [2] SHEN X B, XU J Y, WEN J X. Phenomenological characteristics of hydrogen/air premixed flame propagation in closed rectangular channels [J]. Renewable Energy, 2021, 174: 606–615. doi: 10.1016/j.renene.2021.04.056 [3] CAO W G, LIU Y F, CHEN R K, et al. Pressure release characteristics of premixed hydrogen-air mixtures in an explosion venting device with a duct [J]. International Journal of Hydrogen Energy, 2021, 46(12): 8810–8819. doi: 10.1016/j.ijhydene.2020.12.052 [4] SAN M C, HECHT E S, EKOTO I W, et al. Overview of the DOE hydrogen safety, codes and standards program, part 3: advances in research and development to enhance the scientific basis for hydrogen regulations, codes and standards [J]. International Journal of Hydrogen Energy, 2017, 42(11): 7263–7274. doi: 10.1016/j.ijhydene.2016.07.014 [5] ABE J O, POPOOLA A P I, AJENIFUJA E, et al. Hydrogen energy, economy and storage: review and recommendation [J]. International Journal of Hydrogen Energy, 2019, 44(29): 15072–15086. doi: 10.1016/j.ijhydene.2019.04.068 [6] 路长, 李毅, 潘荣锟. 管道氢气-空气预混气体爆炸特征的试验研究 [J]. 安全与环境学报, 2016, 16(3): 38–42. doi: 10.13637/j.issn.1009-6094.2016.03.008LU C, LI Y, PAN R K. Experimental study on explosion characteristics of hydrogen-air premixed gas in pipelines [J]. Journal of Safety and Environment, 2016, 16(3): 38–42. doi: 10.13637/j.issn.1009-6094.2016.03.008 [7] TROIANI G. Effect of velocity inflow conditions on the stability of a CH4/air jet-flame [J]. Combustion and Flame, 2009, 156(2): 539–542. doi: 10.1016/j.combustflame.2008.11.020 [8] NISHIMURA I, MOGI T, DOBASHI R. Simple method for predicting pressure behavior during gas explosions in confined spaces considering flame instabilities [J]. Journal of Loss Prevention in the Process Industries, 2013, 26(2): 351–354. doi: 10.1016/j.jlp.2011.08.009 [9] HOLBORN P G, BATTERSBY P N, INGRAIN J M, et al. Modelling the mitigation of a hydrogen deflagration in a nuclear waste silo ullage with water fog [J]. Process Safety and Environmental Protection, 2013, 91(6): 476–482. doi: 10.1016/j.psep.2012.11.001 [10] WEN X P, WANG M M, SU T F, et al. Suppression effects of ultrafine water mist on hydrogen/methane mixture explosion in an obstructed chamber [J]. International Journal of Hydrogen Energy, 2019, 44(60): 32332–32342. doi: 10.1016/j.ijhydene.2019.10.110 [11] DIXON-LEWIS G, MARSHALL P, RUSCIC B, et al. Inhibition of hydrogen oxidation by HBr and Br2 [J]. Combustion and Flame, 2012, 159(2): 528–540. doi: 10.1016/j.combustflame.2011.08.016 [12] SIKES T, MATHIEU O, KULATILAKA W D, et al. Laminar flame speeds of DEMP, DMMP, and TEP added to H2- and CH4-air mixtures [J]. Proceedings of the Combustion Institute, 2019, 37(3): 3775–3781. doi: 10.1016/j.proci.2018.05.042 [13] YAN C, LI M S, BI L H, et al. Hydrogen cloud explosion evaluation under inert gas atmosphere [J]. Fuel Processing Technology, 2018, 180: 96–104. doi: 10.1016/j.fuproc.2018.08.015 [14] WEI H Q, XU Z L, ZHOU L, et al. Effect of hydrogen-air mixture diluted with argon/nitrogen/carbon dioxide on combustion processes in confined space [J]. International Journal of Hydrogen Energy, 2018, 43(31): 14798–14805. doi: 10.1016/j.ijhydene.2018.06.038 [15] YANG Z K, ZHAO K, SONG X Z, et al. Effects of mesh aluminium alloys and propane addition on the explosion-suppression characteristics of hydrogen-air mixture [J]. International Journal of Hydrogen Energy, 2021, 46(70): 34998–35013. doi: 10.1016/j.ijhydene.2021.08.035 [16] ZHOU S Y, GAO J C, LUO Z M, et al. Effects of mesh aluminium alloy and aluminium velvet on the explosion of H2/air, CH4/air and C2H2/air mixtures [J]. International Journal of Hydrogen Energy, 2021, 46(27): 14871–14880. doi: 10.1016/j.ijhydene.2021.01.200 [17] PANG L, WANG C, HAN M, et al. A study on the characteristics of the deflagration of hydrogen-air mixture under the effect of a mesh aluminum alloy [J]. Journal of Hazardous Materials, 2015, 299: 174–180. doi: 10.1016/j.jhazmat.2015.06.027 [18] SONG X Z, ZUO X C, YANG Z K, et al. The explosion-suppression performance of mesh aluminum alloys and spherical nonmetallic materials on hydrogen-air mixtures [J]. International Journal of Hydrogen Energy, 2020, 45(56): 32686–32701. doi: 10.1016/j.ijhydene.2020.08.197 [19] FARADAY M. LXIV. On the diamagnetic conditions of flame and gases [J]. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 1847, 31(210): 401–421. doi: 10.1080/14786444708645886 [20] KUMAR M, AGARWAL S, KUMAR V, et al. Experimental investigation on butane diffusion flames under the influence of magnetic field by using digital speckle pattern interferometry [J]. Applied Optics, 2015, 54(9): 2450–60. doi: 10.1364/AO.54.002450 [21] AGARWAL S, KUMAR M, SHAKHER C. Experimental investigation of the effect of magnetic field on temperature and temperature profile of diffusion flame using circular grating Talbot interferometer [J]. Optics and Lasers in Engineering, 2015, 68: 214–221. doi: 10.1016/j.optlaseng.2015.01.004 [22] ITOH S, SHINODA M, KITAGAWA K, et al. Spatially resolved elemental analysis of a hydrogen-air diffusion flame by laser-induced plasma spectroscopy (LIPS) [J]. Microchemical Journal, 2001, 70(2): 143–152. doi: 10.1016/S0026-265X(01)00107-2 [23] YAMADA E, SHINODA M, YAMASHITA H, et al. Experimental and numerical analyses of magnetic effect on OH radical distribution in a hydrogen-oxygen diffusion flame [J]. Combustion and Flame, 2003, 135: 365–379. doi: 10.1016/j.combustflame.2003.08.005 [24] YAMADA E, SHINODA M, YAMASHITA H, et al. Influence of four kinds of gradient magnetic fields on hydrogen-oxygen flame [J]. AIAA Journal, 2003, 41(8): 1535–1541. doi: 10.2514/2.2104 [25] 高建村, 王乐, 胡守涛, 等. 不同磁性金属丝对丙烷爆炸反应抑制机理研究 [J]. 中国安全生产科学技术, 2020, 16(7): 125–130. doi: 10.11731/j.issn.1673-193x.2020.07.020GAO J C, WANG L, HU S T, et al. Inhibition mechanism of different magnetic metal wires on propane explosion reaction [J]. China Safety Production Science and Technology, 2020, 16(7): 125–130. doi: 10.11731/j.issn.1673-193x.2020.07.020 [26] 高建村, 杨喜港, 胡守涛, 等. 外加磁场对乙炔气体爆炸反应影响研究 [J]. 爆炸与冲击, 2022, 42(7): 075401.GAO J C, YANG X G, HU S T, et al. Effect of external magnetic field on acetylene gas explosion reaction [J]. Explosion and Shock Waves, 2022, 42(7): 075401. [27] 黄晓东, 王晓兵, 梅建, 等. 汽车加油(气)站、轻质燃油和液化石油气汽车罐车用阻隔防爆储罐技术要求: AQ 3001—2005 [S]. 北京: 国家安全生产监督管理总局, 2005. [28] 黄晓东, 王晓兵, 梅建, 等. 阻隔防爆撬装式汽车加油(气)装置技术要求: AQ 3002—2005 [S]. 北京: 国家安全生产监督管理总局, 2005. [29] WANG H, YOU X Q, JOSHI A V, et al. USC mech version Ⅱ. high-temperature combustion reaction model of H2/CO/C1-C4 compounds [DB/OL]. [2022-06-15]. http://ignis.usc.edu/USC_Mech_II.htm, 2007. -






 下载:
下载: