Theoretical Study on the Polymerization Mechanism of Hydrogen-Doped Carbon Monoxide under High Pressure
-
摘要: 一氧化碳(CO)是典型的低原子序数(Z)体系,能够在高压下通过聚合反应形成聚一氧化碳(p-CO),其高压聚合机理与结构对于理解压力诱导成键机制和探索新型功能材料具有重要价值,但是相关研究受到CO聚合压力偏高和产物p-CO在常压下具有亚稳态特性两方面阻碍。目前,人们发现氢气(H2)掺杂有助于CO聚合,但是对相应的聚合机理和产物结构缺乏认知,为此,利用分子动力学方法研究了H2掺杂对CO高压聚合机理的影响规律。结果表明,摩尔分数为10%的H2掺杂具有降低CO聚合压力的最优效果。当压力为3~4 GPa时,H2通过物理作用促进了CO的二聚化;当压力提高到5 GPa时,H2由于化学惰性阻碍了体系的进一步聚合;当压力提高到10 GPa时,H2能够参与聚合反应,产生C―H键和O―H键。最终,聚合反应会形成一个无序、以C―C键和C=O键为主的三维网状结构p-CO/H。Abstract: Carbon monoxide (CO), as a prototypical low-Z molecular system, can polymerize under high pressure to form polymeric carbon monoxide (p-CO). The polymerization mechanisms and structures are of fundamental importance for understanding pressure-induced bonding and exploring novel functional materials. However, progress in this field has been hindered by two major challenges: the high-pressure requirements for CO and the metastable property of p-CO at ambient pressure. Recent studies have shown that hydrogen (H2) doping can facilitate the polymerization of CO, but the polymerization mechanisms and structures are still poorly understood. In this work, molecular dynamics simulations were performed to investigate the influence of H2 on the polymerization progress of CO. The results reveal that a doping ratio of 10% can optimally reduce the polymerization pressure of CO. At 3–4 GPa, H2 physically induces the dimerization reaction of CO. At 5 GPa, the chemical inertness of H2 inhibits further polymerization of CO. When the pressure reaches 10 GPa, H2 participates in the polymerization reaction, forming C―H and O―H bonds. Finally, the polymerization produces a disordered three-dimensional network structure (p-CO/H) dominated by C―C and C=O bonds.
-
Key words:
- carbon monoxide /
- hydrogen /
- high-pressure polymerization /
- molecular dynamics
-
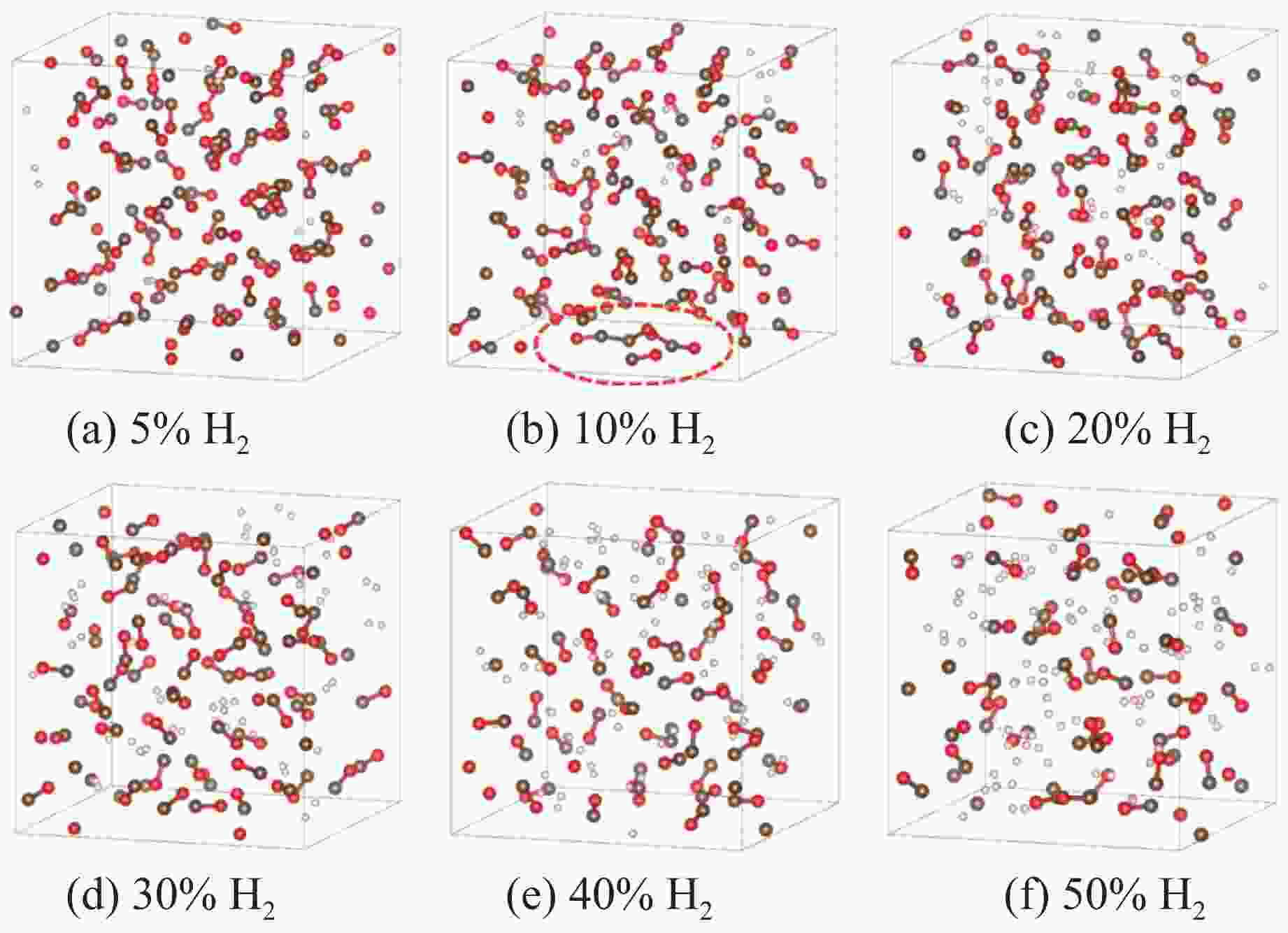
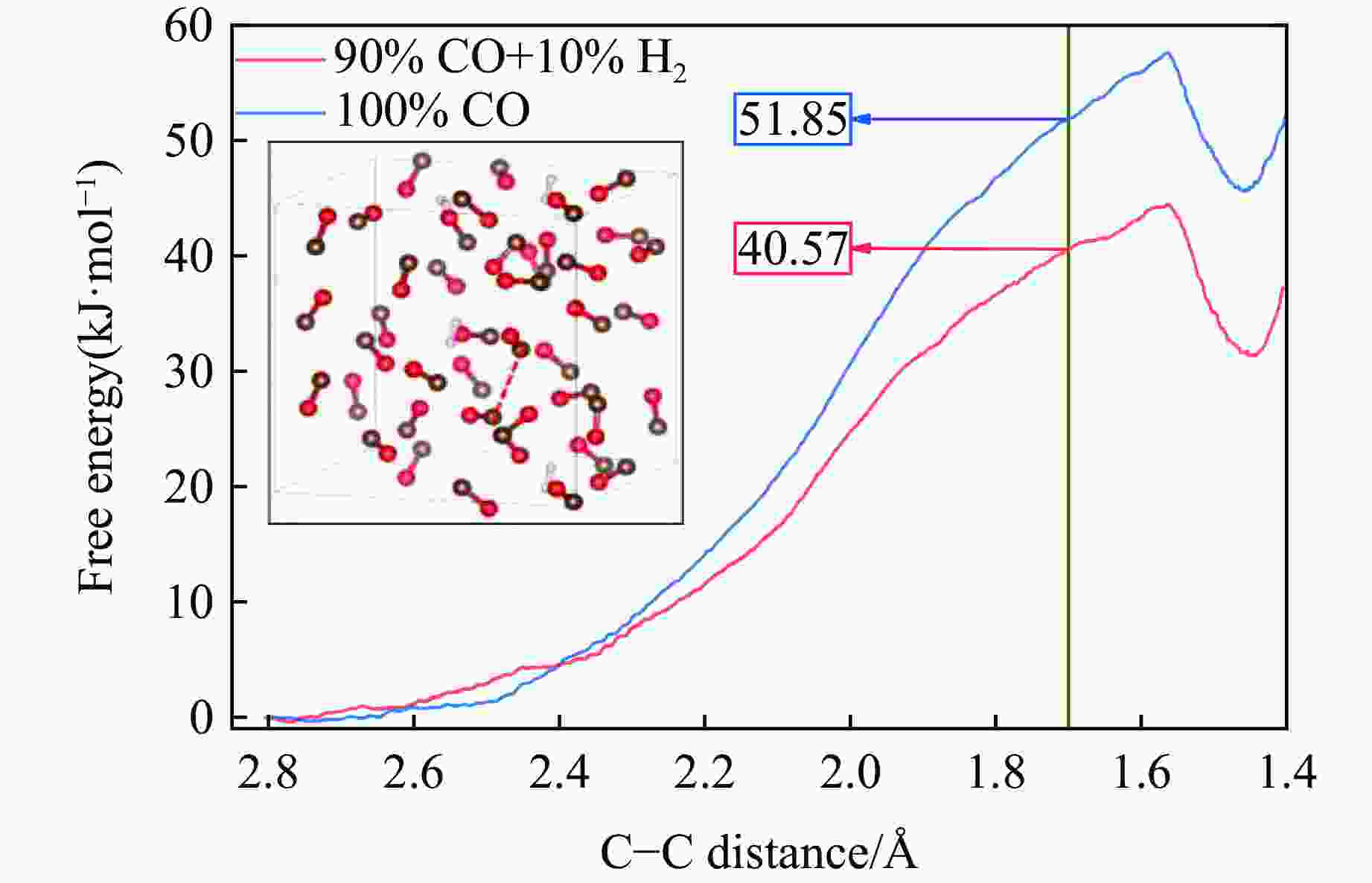
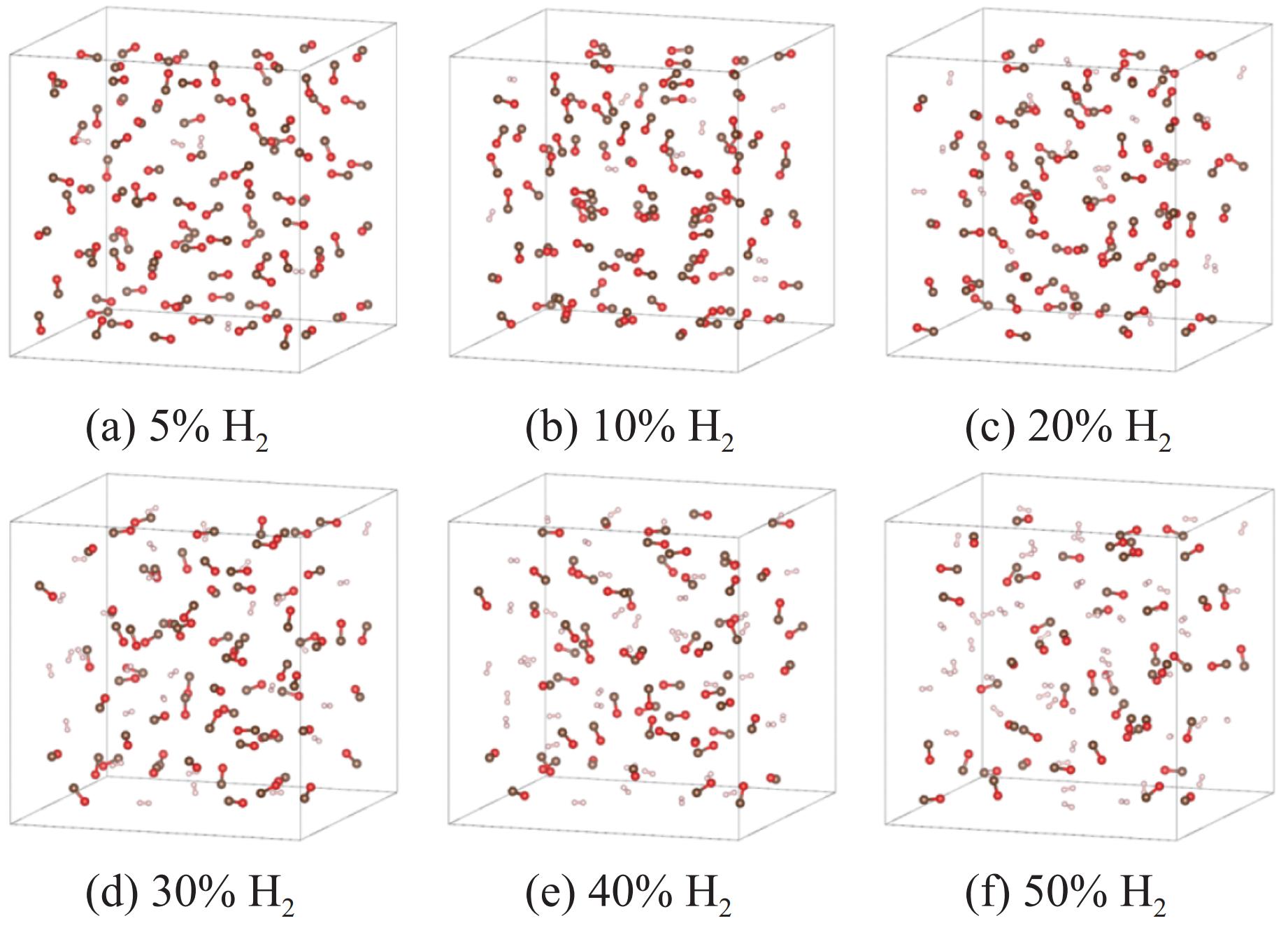
图 3 3 GPa下、充分弛豫后不同H2摩尔分数的H2/CO混合体系结构(褐色球为C,红色球为O,白色球为H;红圈表示CO寡聚体;为简洁起见,隐藏了H―H键)
Figure 3. Structural models of H2/CO mixed systems with different H2 molarratios under 3 GPa (The brown, red and white balls denote C, O and H atoms, respectively; the red circle indicates CO oligomer; the H―H bonds are omitted for clarity.)
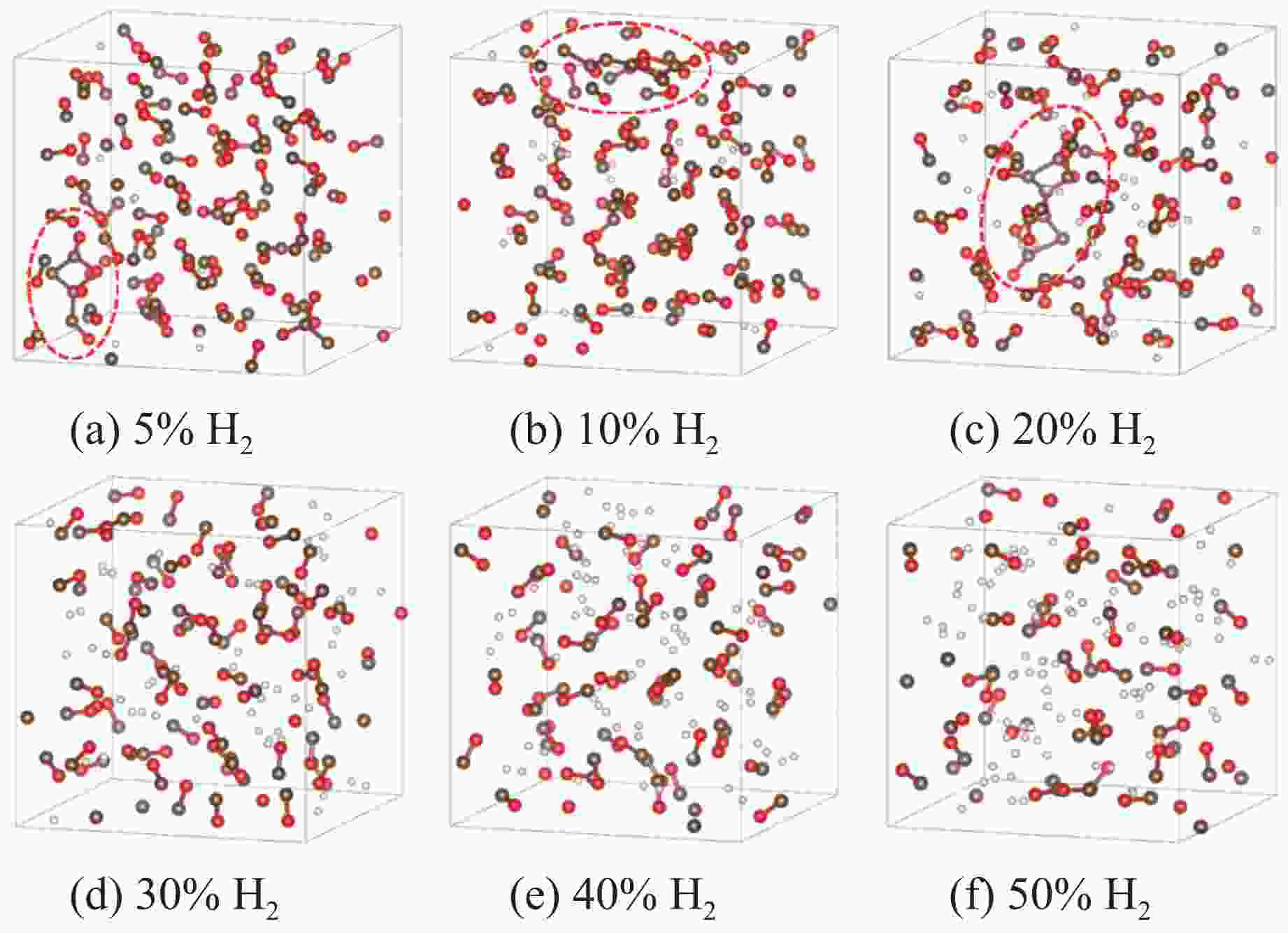
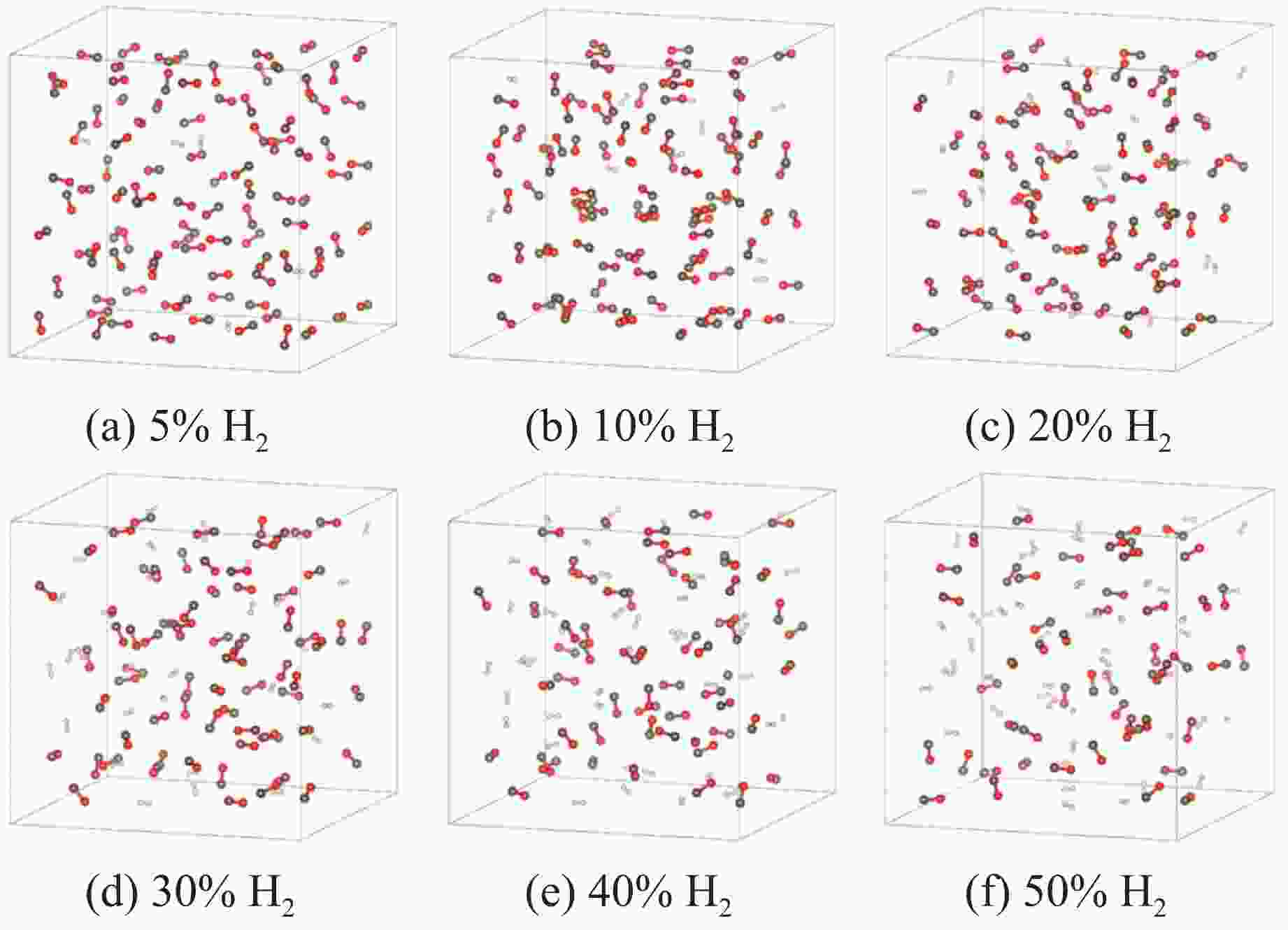
图 4 4 GPa下、充分弛豫后不同H2摩尔分数的H2/CO混合体系结构(褐色球为C,红色球为O,白色球为H;红圈表示CO寡聚体;为简洁起见,隐藏了H―H键)
Figure 4. Structural models of H2/CO mixed systems with different H2 molarratios under 4 GPa (The brown, red and white balls denote C, O and H atoms, respectively; the red circle indicates CO oligomer; the H―H bonds are omitted for clarity.)
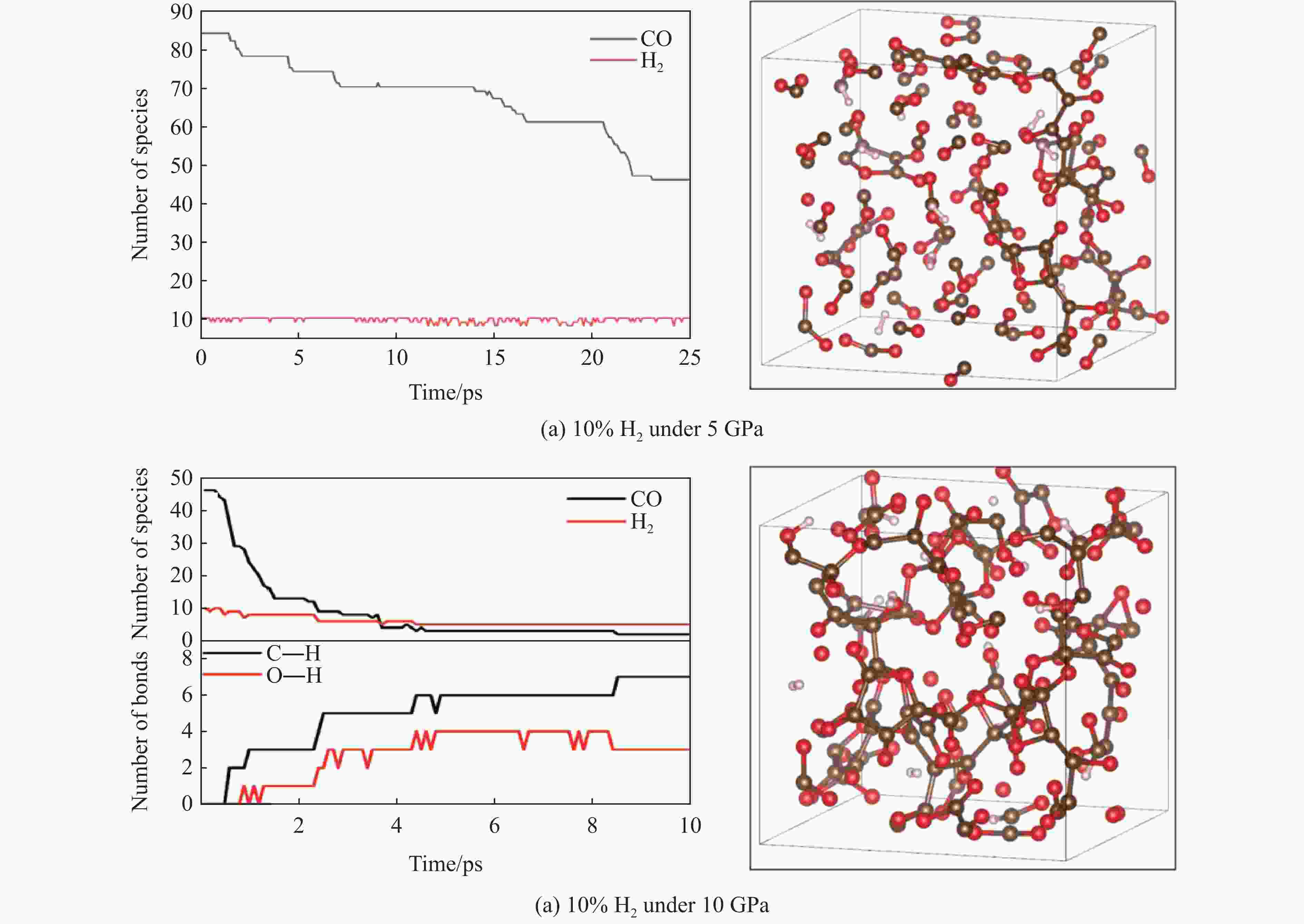
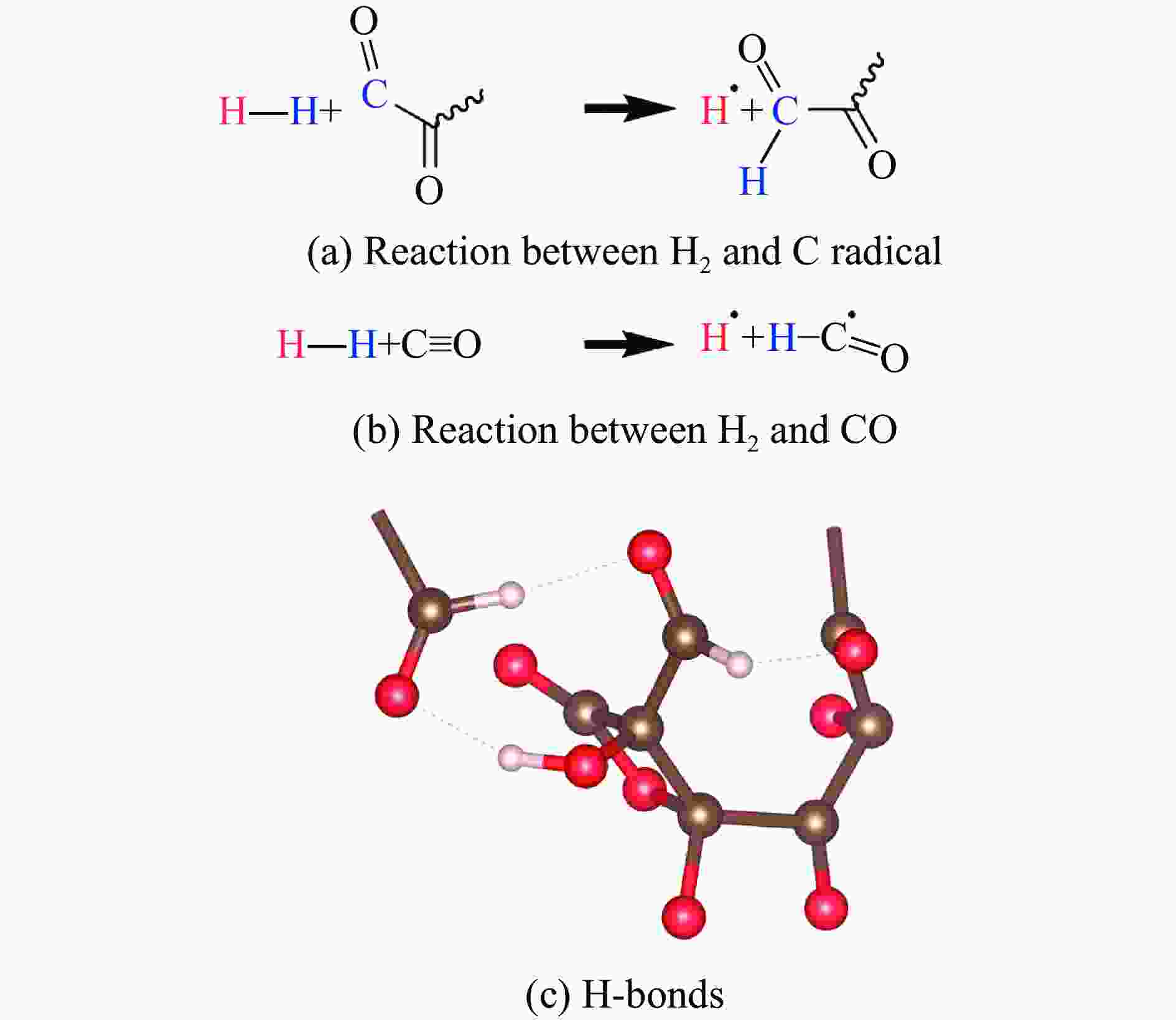
图 9 10 GPa压力下10% H2掺杂CO体系中与H2相关的化学反应以及形成的H键(褐色球为C,红色球为O,白色球为H,波浪线表示与聚合网络连接的化学键)
Figure 9. Chemical reactions involved in H2 and H-bonds in the mixed system under 10 GPa (Brown, red and white balls denote C, O and H atoms, respectively. The wavy lines represent the chemical bonds connected to the aggregated network.)
-
[1] LEONHARDI T C, MILITZER B. Ab initio simulations of liquid carbon monoxide at high pressure [J]. High Energy Density Physics, 2017, 22: 41–45. doi: 10.1016/j.hedp.2017.02.005 [2] SHI Y, WANG J Z, ZHANG Z Y, et al. Carbon monoxide in an extremely metal-poor galaxy [J]. Nature Communications, 2016, 7: 13789. doi: 10.1038/ncomms13789 [3] EREMETS M I, STRUZHKIN V V, MAO H K, et al. Exploring superconductivity in low-Z materials at megabar pressures [J]. Physica B: Condensed Matter, 2003, 329: 1312–1316. [4] YAMANAKA S, KINI N S, KUBO A, et al. Topochemical 3D polymerization of C60 under high pressure at elevated temperatures [J]. Journal of the American Chemical Society, 2008, 130(13): 4303–4309. doi: 10.1021/ja076761k [5] IOTA V, YOO C S, CYNN H. Quartzlike carbon dioxide: an optically nonlinear extended solid at high pressures and temperatures [J]. Science, 1999, 283(5407): 1510–1513. doi: 10.1126/science.283.5407.1510 [6] EREMETS M I, GAVRILIUK A G, TROJAN I A, et al. Single-bonded cubic form of nitrogen [J]. Nature Materials, 2004, 3(8): 558–563. doi: 10.1038/nmat1146 [7] MAO H K, JI C, LI B, et al. Extreme energetic materials at ultrahigh pressures [J]. Engineering, 2020, 6(9): 976–980. doi: 10.1016/j.eng.2020.07.010 [8] YOO C S. Chemistry under extreme conditions: pressure evolution of chemical bonding and structure in dense solids [J]. Matter and Radiation at Extremes, 2020, 5(1): 018202. doi: 10.1063/1.5127897 [9] LIPP M, EVANS W J, GARCIA-BAONZA V, et al. Carbon monoxide: spectroscopic characterization of the high-pressure polymerized phase [J]. Journal of Low Temperature Physics, 1998, 111(3): 247–256. doi: 10.1023/A:1022267115640 [10] SUN J, KLUG D D, PICKARD C J, et al. Controlling the bonding and band gaps of solid carbon monoxide with pressure [J]. Physical Review Letters, 2011, 106(14): 145502. doi: 10.1103/PhysRevLett.106.145502 [11] BATYREV I G, MATTSON W D, RICE B M. Modeling of a random network of extended CO solids [J]. AIP Conference Proceedings, 2012, 1426(1): 717–720. doi: 10.1063/1.3686379 [12] XIA K, SUN J, PICKARD C J, et al. Ground state structure of high-energy-density polymeric carbon monoxide [J]. Physical Review B, 2017, 95(14): 144102. doi: 10.1103/PhysRevB.95.144102 [13] SUN C L, GUO W, ZHU J L, et al. High-energy-density polymeric carbon oxide: layered CxOy solids under pressure [J]. Physical Review B, 2021, 104(9): 094102. doi: 10.1103/PhysRevB.104.094102 [14] HUANG X, JIAO F B, ZHANG C Y, et al. Investigation of polymeric CO synthesized at high pressure and its stability under ambient conditions: a first-principles study [J]. The Journal of Physical Chemistry C, 2022, 126(46): 19571–19579. doi: 10.1021/acs.jpcc.2c04467 [15] KONDRIN M V, LEBED Y B, BRAZHKIN V V. A new polymorph of graphene monoxide: an all-sp3 bonded metal and ambient pressure superconductor [J]. CrystEngComm, 2023, 25(9): 1328–1332. doi: 10.1039/D2CE01561G [16] SUN S H, XU J J, GOU H Y, et al. Pressure-induced in situ construction of p-CO/HNIW explosive composites with excellent laser initiation and detonation performance [J]. ACS Applied Materials & Interfaces, 2021, 13(17): 20718–20727. doi: 10.1021/acsami.1c03856 [17] MILLS R L, SCHIFERL D, KATZ A I, et al. New phases and chemical reactions in solid CO under pressure [J]. Journal de Physique Colloques, 1984, 45(C8): 189–190. doi: 10.1051/jphyscol:1984833 [18] DANG N C, CIEZAK-JENKINS J A. Kinetic effects on the morphology and stability of the pressure-induced extended-solid of carbon monoxide [J]. The Journal of Chemical Physics, 2018, 148(14): 144702. doi: 10.1063/1.5004556 [19] EVANS W J, LIPP M J, YOO C S, et al. Pressure-induced polymerization of carbon monoxide: disproportionation and synthesis of an energetic lactonic polymer [J]. Chemistry of Materials, 2006, 18(10): 2520–2531. doi: 10.1021/cm0524446 [20] SHIEH S R, JARRIGE I, WU M, et al. Electronic structure of carbon dioxide under pressure and insights into the molecular-to-nonmolecular transition [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(46): 18402–18406. doi: 10.1073/pnas.1305116110 [21] RYU Y J, KIM M, LIM J, et al. Dense carbon monoxide to 160 GPa: stepwise polymerization to two-dimensional layered solid [J]. The Journal of Physical Chemistry C, 2016, 120(48): 27548–27554. doi: 10.1021/acs.jpcc.6b09434 [22] LIPP M J, EVANS W J, BAER B J, et al. High-energy-density extended CO solid [J]. Nature Materials, 2005, 4(3): 211–215. doi: 10.1038/nmat1321 [23] CEPPATELLI M, SERDYUKOV A, BINI R, et al. Pressure induced reactivity of solid CO by FTIR studies [J]. The Journal of Physical Chemistry B, 2009, 113(19): 6652–6660. doi: 10.1021/jp900586a [24] LI X Y, PENG Z H, JIANG C W, et al. Insights into the structure and polymerization mechanisms of CO molecules under pressure [J]. Progress in Solid State Chemistry, 2024, 76: 100491. doi: 10.1016/j.progsolidstchem.2024.100491 [25] SANTORO M, BINI R, CEPPATELLI M, et al. High pressure structural changes in amorphous polymeric carbon monoxide by combined infrared spectroscopy and X-ray diffraction [J]. The Journal of Physical Chemistry C, 2022, 126(28): 11840–11845. doi: 10.1021/acs.jpcc.2c03204 [26] SCELTA D, CEPPATELLI M, BINI R, et al. High temperature decomposition of polymeric carbon monoxide at pressures up to 120 GPa [J]. The Journal of Chemical Physics, 2023, 159(8): 084501. doi: 10.1063/5.0157907 [27] SANTORO M, DZIUBEK K, SCELTA D, et al. High pressure synthesis of all-transoid polycarbonyl [―(C=O)―]n in a zeolite [J]. Chemistry of Materials, 2015, 27(19): 6486–6489. doi: 10.1021/acs.chemmater.5b02596 [28] SANTORO M, SCELTA D, DZIUBEK K, et al. Synthesis of 1D polymer/zeolite nanocomposites under high pressure [J]. Chemistry of Materials, 2016, 28(11): 4065–4071. doi: 10.1021/acs.chemmater.6b01639 [29] RADEMACHER N, BAYARJARGAL L, MORGENROTH W, et al. The local atomic structures of liquid CO at 3.6 GPa and polymerized CO at 0 to 30 GPa from high-pressure pair distribution function analysis [J]. Chemistry—A European Journal, 2014, 20(36): 11531–11539. doi: 10.1002/chem.201403000 [30] YANG Y P, CHENG P, ZHANG S L, et al. Theoretical insights into the CO dimerization and trimerization on Pt nanocluster [J]. RSC Advances, 2016, 6(6): 4354–4364. doi: 10.1039/C5RA25989D [31] YANG Y P, CHENG P, HUANG S P. Theoretical study on the catalysis effect of platinum cluster during carbon monoxide polymer growth [J]. ChemistrySelect, 2017, 2(6): 2150–2158. doi: 10.1002/slct.201601699 [32] RYU Y J, YOO C S, KIM M, et al. Hydrogen-doped polymeric carbon monoxide at high pressure [J]. The Journal of Physical Chemistry C, 2017, 121(18): 10078–10086. doi: 10.1021/acs.jpcc.7b01506 [33] RYU Y J, YOO C S, LIM J, et al. High-density COHX network glass [J]. The Journal of Physical Chemistry C, 2020, 124(1): 107–114. doi: 10.1021/acs.jpcc.9b09479 [34] MARTÍNEZ L, ANDRADE R, BIRGIN E G, et al. PACKMOL: a package for building initial configurations for molecular dynamics simulations [J]. Journal of Computational Chemistry, 2009, 30(13): 2157–2164. doi: 10.1002/jcc.21224 [35] VANDEVONDELE J, KRACK M, MOHAMED F, et al. QUICKSTEP: fast and accurate density functional calculations using a mixed gaussian and plane waves approach [J]. Computer Physics Communications, 2005, 167(2): 103–128. doi: 10.1016/j.cpc.2004.12.014 [36] LIPPERT G, HUTTER J, PARRINELLO M. A hybrid Gaussian and plane wave density functional scheme [J]. Molecular Physics, 1997, 92(3): 477–488. doi: 10.1080/002689797170220 [37] VANDEVONDELE J, HUTTER J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases [J]. The Journal of Chemical Physics, 2007, 127(11): 114105. doi: 10.1063/1.2770708 [38] GRIMME S, ANTONY J, EHRLICH S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu [J]. The Journal of Chemical Physics, 2010, 132(15): 154104. doi: 10.1063/1.3382344 [39] BUSSI G, DONADIO D, PARRINELLO M. Canonical sampling through velocity rescaling [J]. The Journal of Chemical Physics, 2007, 126(1): 014101. doi: 10.1063/1.2408420 [40] MOMMA K, IZUMI F. Vesta 3 for three-dimensional visualization of crystal, volumetric and morphology data [J]. Journal of Applied Crystallography, 2011, 44(6): 1272–1276. doi: 10.1107/S0021889811038970 [41] HUMPHREY W, DALKE A, SCHULTEN K. VMD: visual molecular dynamics [J]. Journal of Molecular Graphics, 1996, 14(1): 33–38. doi: 10.1016/0263-7855(96)00018-5 [42] KRESSE G, HAFNER J. Ab initio molecular dynamics for liquid metals [J]. Physical Review B, 1993, 47(1): 558–561. doi: 10.1103/PhysRevB.47.558 [43] KRESSE G, FURTHMÜLLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set [J]. Physical Review B, 1996, 54(16): 11169. doi: 10.1103/PhysRevB.54.11169 [44] KRESSE G, JOUBERT D. From ultrasoft pseudopotentials to the projector augmented-wave method [J]. Physical Review B, 1999, 59(3): 1758–1775. doi: 10.1103/PhysRevB.59.1758 [45] WOO T K, MARGL P M, BLÖCHL P E, et al. A combined car-parrinello QM/MM implementation for ab initio molecular dynamics simulations of extended systems: application to transition metal catalysis [J]. The Journal of Physical Chemistry B, 1997, 101(40): 7877–7880. doi: 10.1021/jp9717296 [46] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple [J]. Physical Review Letters, 1996, 77(18): 3865–3868. doi: 10.1103/PhysRevLett.77.3865 -






 下载:
下载: