Crystal Structure and Physical Properties of Sr2He Compound under High Pressure
-
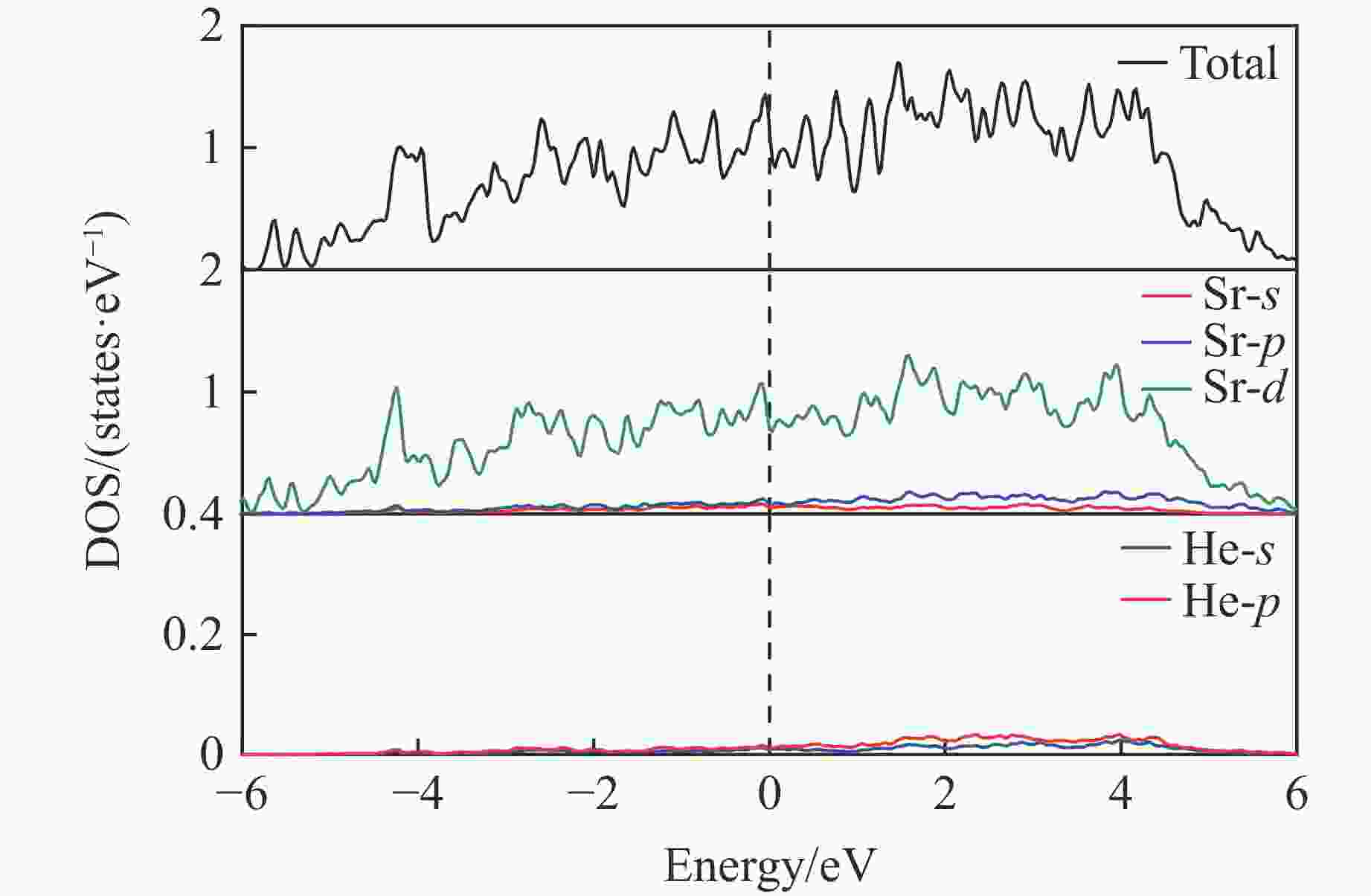
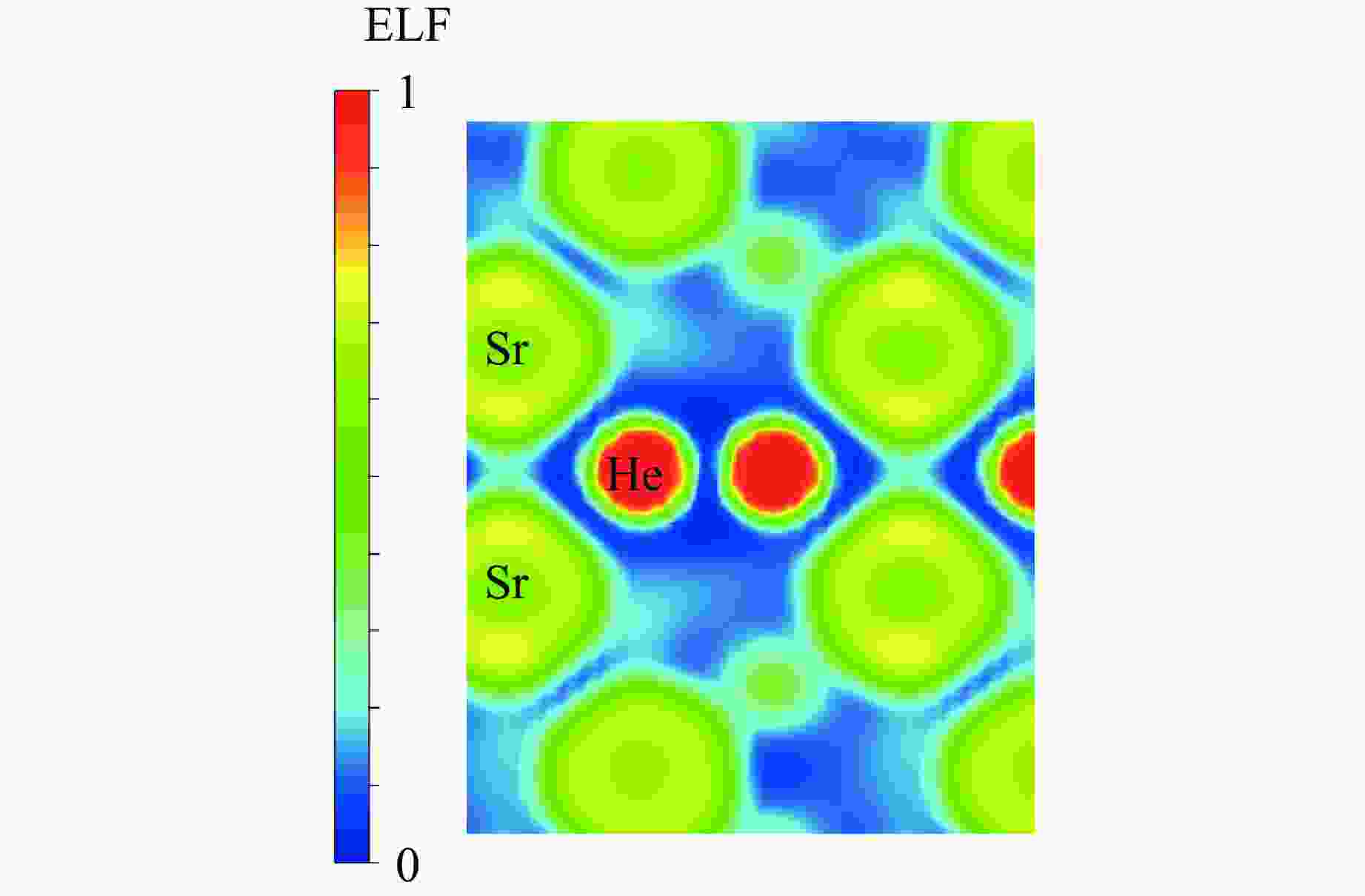
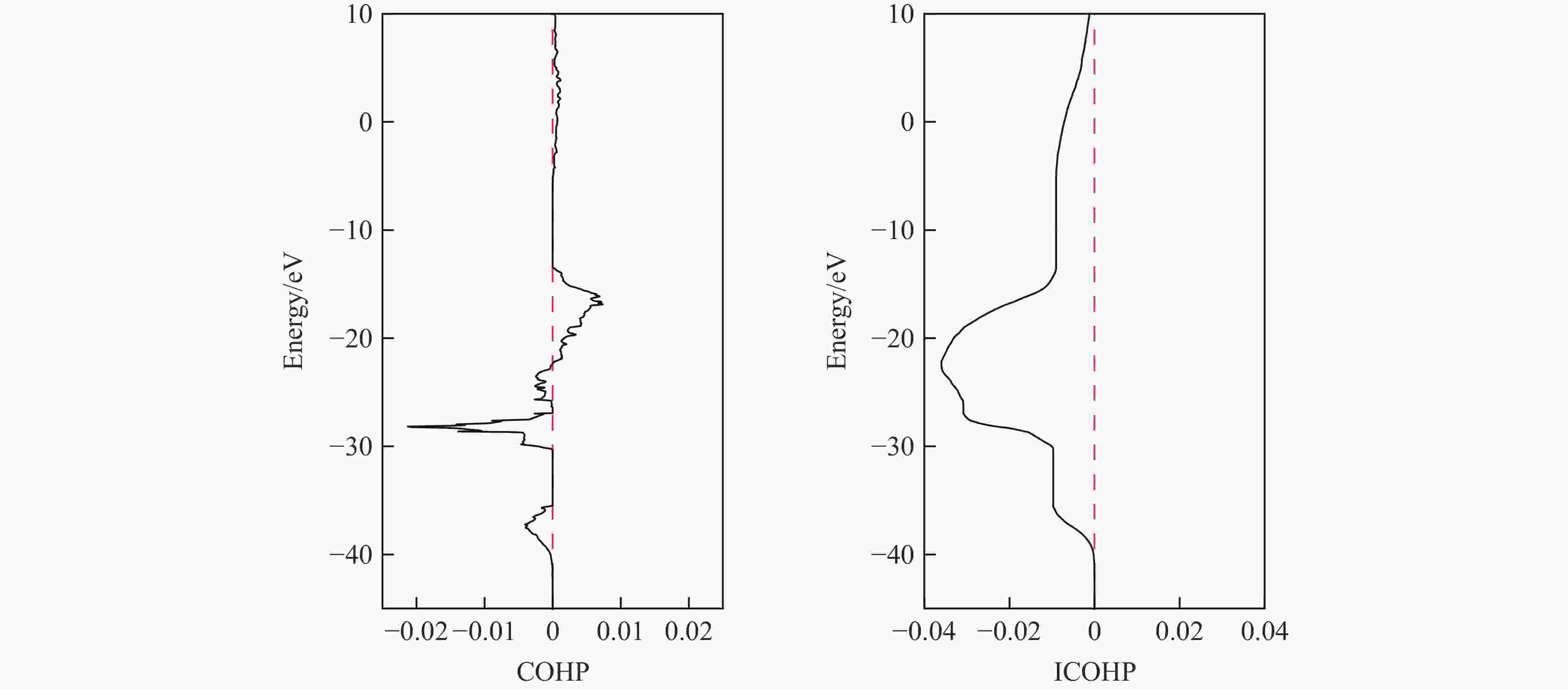
摘要: 采用密度泛函理论框架下的第一性原理计算与CALYPSO晶体结构预测方法相结合,系统探究了惰性元素氦(He)与碱土金属的化合物在高压条件下的结构稳定性。研究发现,在碱土金属中,锶(Sr)与 He 形成的化合物具有相对较低的能量。为此,对400 GPa下Sr2He的晶体结构进行了预测。电子定域函数和态密度分析表明,Sr与He原子之间不存在形成共价键的趋势。此外,Bader电荷分析显示,Sr原子与He原子之间存在离子键作用,电荷从He转移至Sr,从而为阐明Sr2He的成键机制提供了关键见解。研究结果揭示了Sr2He的晶体结构、成键性质及电子特性,为理解此类亚稳材料的稳定性和物理性质提供了理论支撑,并为其实验合成提供了重要指导。Abstract: By combining first-principles calculations under the framework of density functional theory (DFT) and the CALYPSO crystal structure prediction method, the structural stability of the inert element helium (He) and alkaline-earth metals under high-pressure conditions has been systematically investigated. The calculations reveal that among the alkaline-earth metals, strontium (Sr) forms compounds with He exhibiting relatively low energy values. Consequently, the crystal structure of Sr2He at 400 GPa was predicted. Electron localization function (ELF) and density of states (DOS) analyses show no tendency for covalent bond formation between Sr and He atoms. Furthermore, Bader charge analysis reveals ionic bonding between Sr and He atoms, with charge transfer occurring from He to Sr. These results provide key insights into the bonding mechanism of Sr2He. This study elucidates the crystal structure, bonding nature, and electronic properties of Sr2He, offering theoretical support for understanding the stability and physical properties of such metastable materials and providing important guidance for their experimental synthesis.
-
Key words:
- high pressure /
- alkali-earth metals /
- He /
- first-principles calculation /
- CALYPSO /
- structure prediction
-
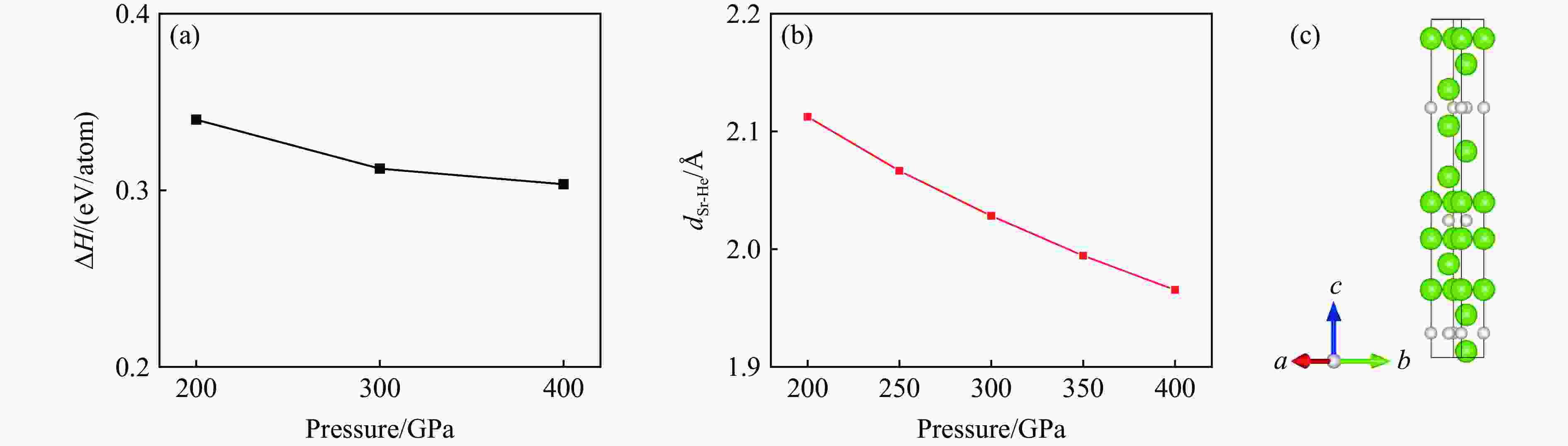
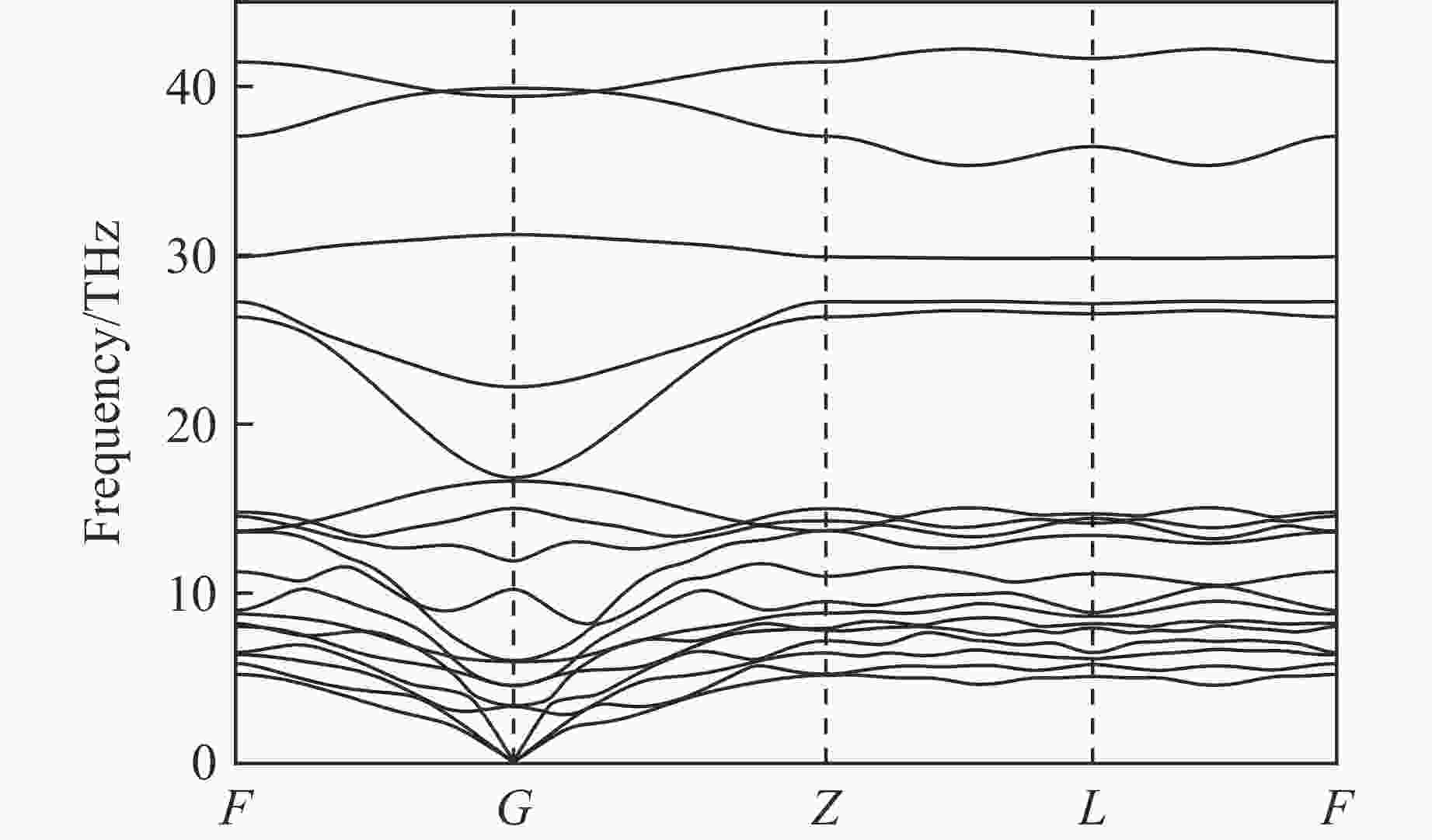
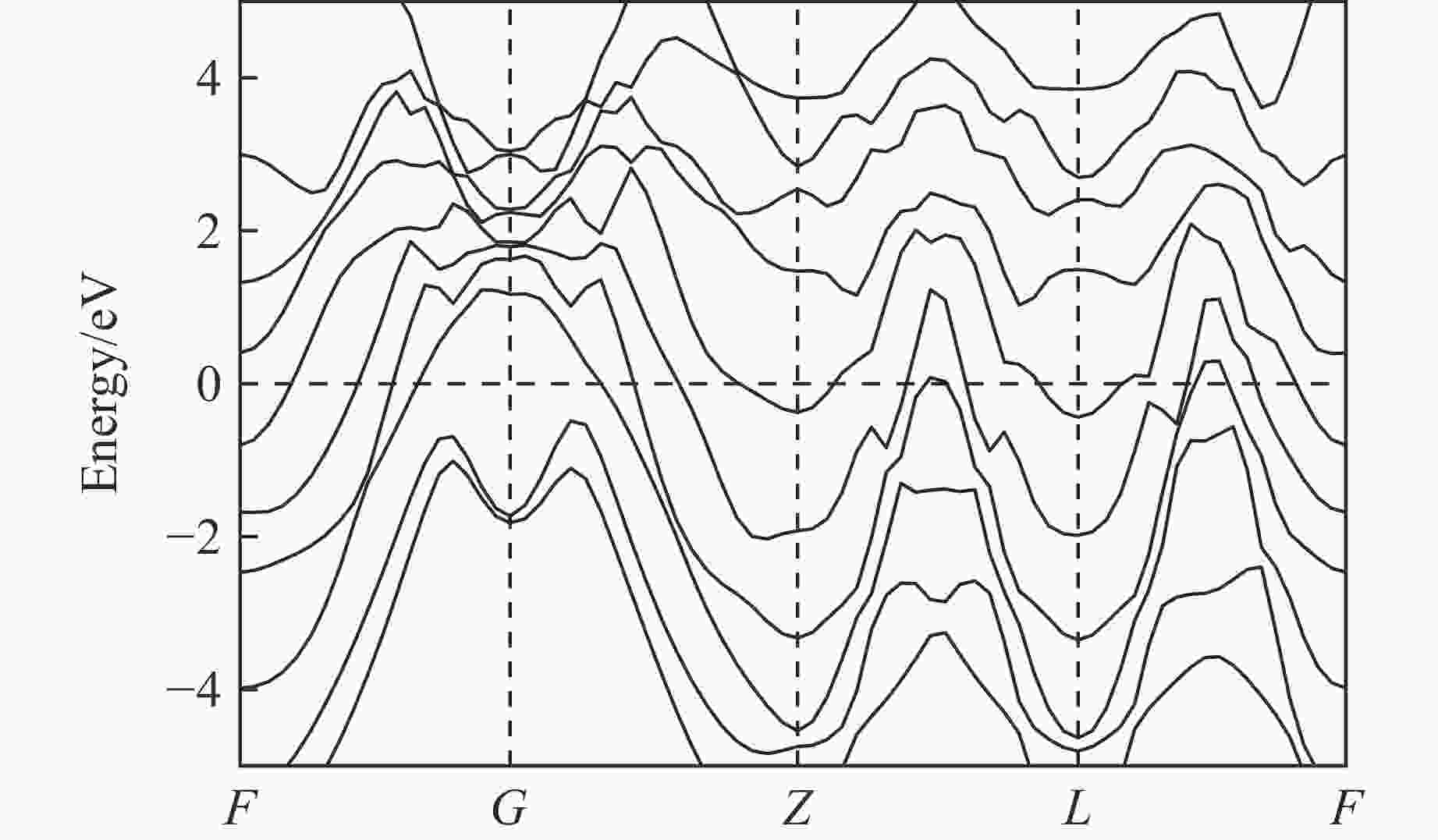
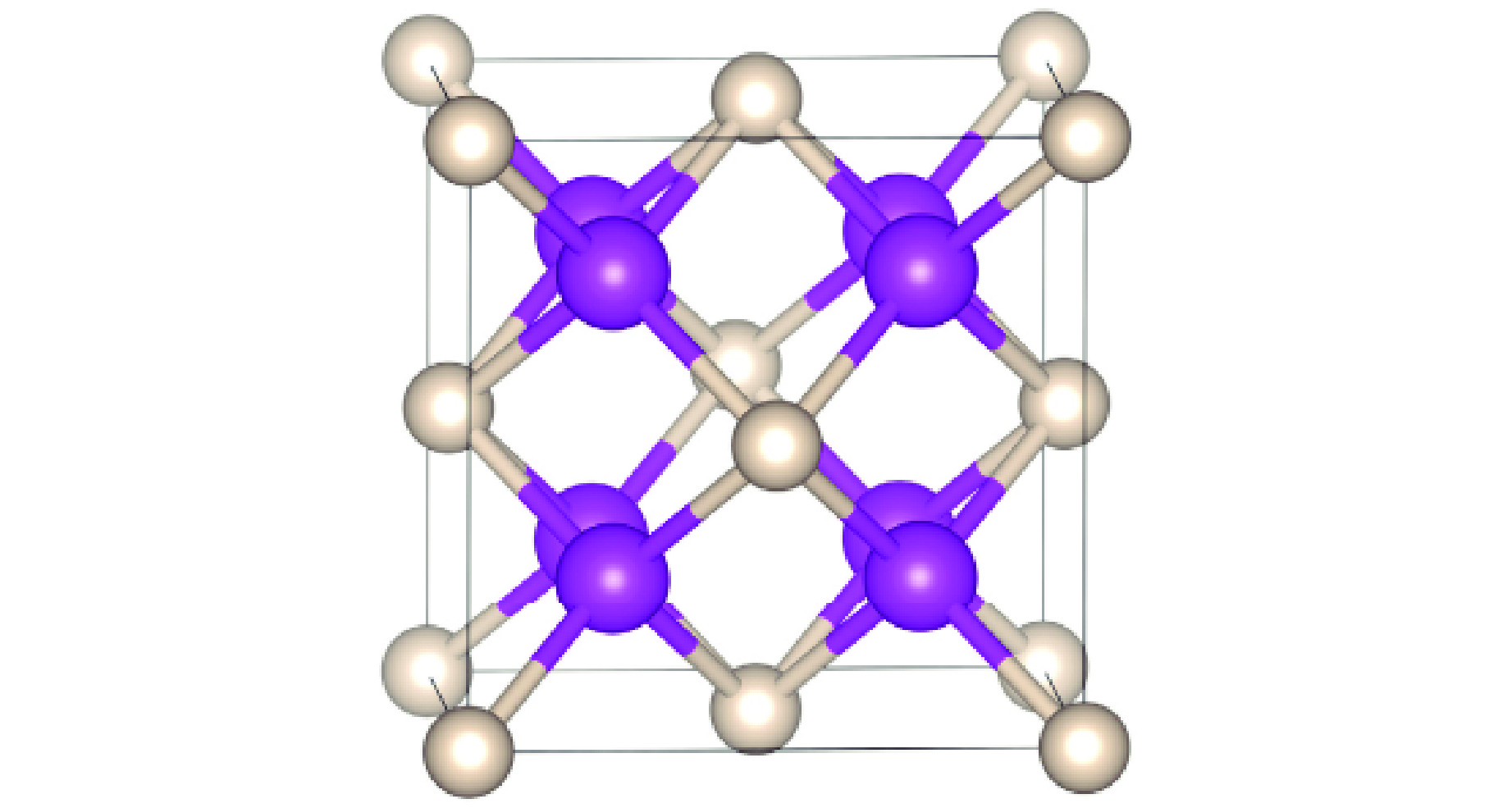
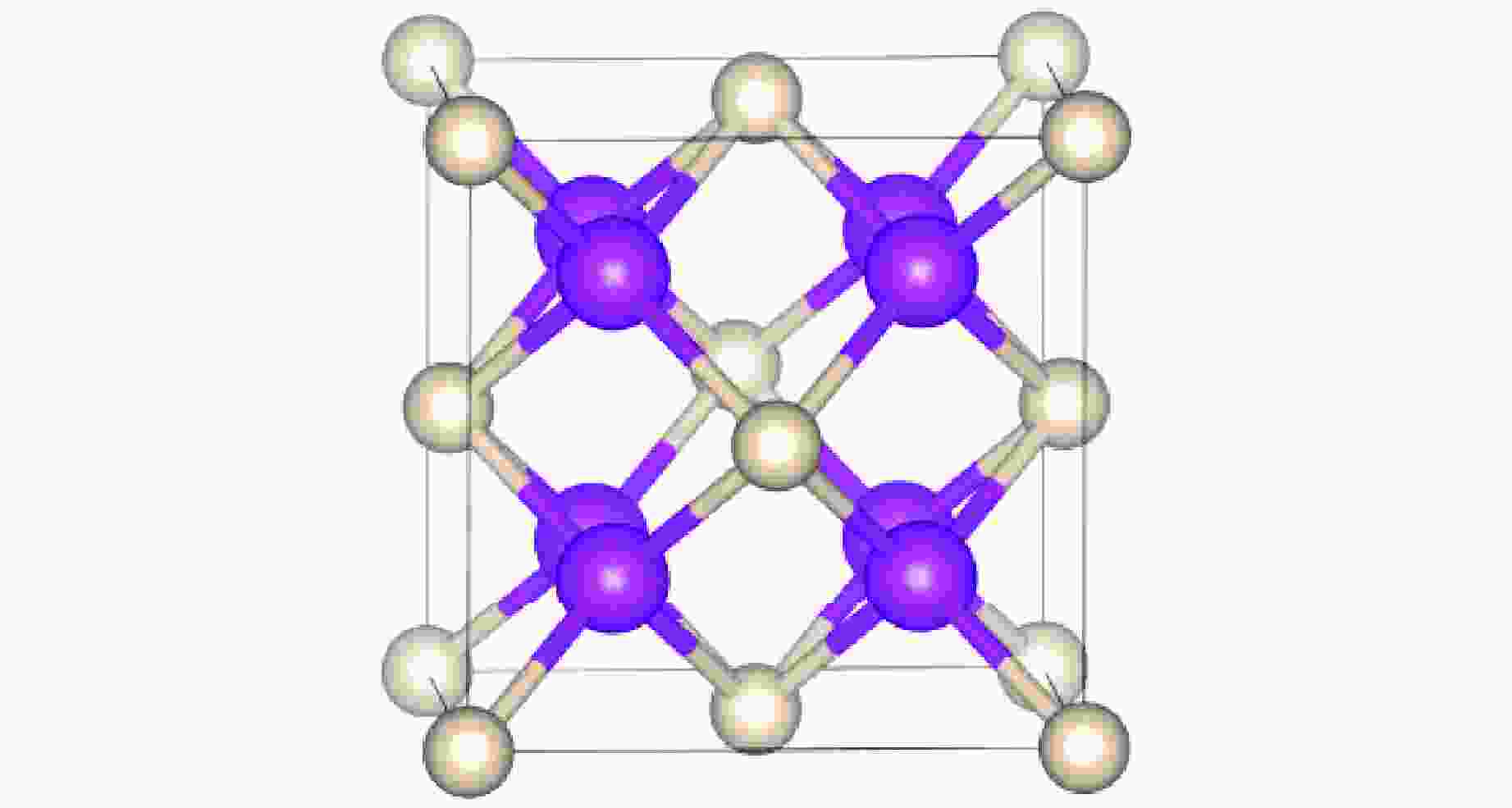
图 2 (a) R3m-Sr2He在200、300和400 GPa下的形成焓,(b) Sr-He原子间距随压力的变化,(c) Sr2He在400 GPa下的晶体结构(绿色和灰色分别代表Sr原子和He原子)
Figure 2. (a) Formation enthalpy of R3m-Sr2He at 200, 300, and 400 GPa; (b) evolution of Sr-He bond distance as a function of pressure; (c) crystal structure of Sr2He at 400 GPa, where green and gray spheres represent Sr and He atoms, respectively
表 1 Ba、Be、Cs、Mg、Rb和Sr元素替换Na后的化合物在200和300 GPa压力下的形成焓
Table 1. Formation enthalpies of Ba, Be, Cs, Mg, Rb and Sr after Na replacement at 200 and 300 GPa
Compound Formation enthalpy/(eV/atom) Compound Formation enthalpy/(eV/atom) 200 GPa 300 GPa 200 GPa 300 GPa Na2He − 0.185692765 − 0.356622880 Mg2He 4.745655837 4.857751318 Ba2He 3.425471253 4.451063222 Rb2He 2.225320695 3.149201765 Be2He 9.452763723 10.218131800 Sr2He 0.339991367 0.312264493 Cs2He 3.940463840 5.399894545 表 2 400 GPa下Sr2He化合物的晶体结构参数
Table 2. Crystal structure parameters of Sr2He compound at 400 GPa
Space group Lattice parameters Wyckoff position R3m a= 2.40880 Å
b=2.40880 Å
c=25.8150 Å
α=β=90°
γ=120°Sr1: 3a ( 0.00000 ,−0.00000 ,0.76818 )Sr2: 3a ( 0.00000 ,−0.00000 ,0.35984 )Sr3: 3a ( 0.00000 ,−0.00000 ,0.25193 )Sr4: 3a ( 0.00000 ,−0.00000 ,0.51049 )He1: 3a ( 0.00000 ,−0.00000 ,0.97281 )He2: 3a ( 0.00000 ,−0.00000 ,0.63903 )表 3 R3m-Sr2He在200~400 GPa下的电荷转移
Table 3. Bader charge transfer values of R3m-Sr2He under pressures of 200–400 GPa
Phase Pressure/GPa Atom Charge/e Sr2He 200 Sr1 0.034740 Sr2 − 0.123743 Sr3 − 0.100587 Sr4 − 0.006534 He1 0.104386 He2 0.091738 Sr2He 300 Sr1 0.030874 Sr2 − 0.115756 Sr3 − 0.103687 Sr4 0.005565 He1 0.097306 He2 0.085698 Sr2He 400 Sr1 0.027394 Sr2 − 0.102514 Sr3 − 0.113609 Sr4 0.013318 He1 0.091454 He2 0.083956 -
[1] BARTLETT N. Xenon hexafluoroplatinate (V) Xe+ [PtF6]– [J]. Proceedings of the Chemical Society London, 1962(6): 197–236. doi: 10.1039/PS9620000197 [2] ZHANG L J, WANG Y C, LV J, et al. Materials discovery at high pressures [J]. Nature Reviews Materials, 2017, 2(4): 17005. doi: 10.1038/natrevmats.2017.5 [3] MIAO M S. Noble gases in solid compounds show a rich display of chemistry with enough pressure [J]. Frontiers in Chemistry, 2020, 8: 570492. doi: 10.3389/fchem.2020.570492 [4] MIAO M S, SUN Y H, ZUREK E, et al. Chemistry under high pressure [J]. Nature Reviews Chemistry, 2020, 4(10): 508–527. doi: 10.1038/s41570-020-0213-0 [5] XU M L, LI Y W, MA Y M. Materials by design at high pressures [J]. Chemical Science, 2022, 13(2): 329–344. doi: 10.1039/D1SC04239D [6] DONG X, OGANOV A R, GONCHAROV A F, et al. A stable compound of helium and sodium at high pressure [J]. Nature Chemistry, 2017, 9(5): 440–445. doi: 10.1038/nchem.2716 [7] ZHANG J R, LV J, LI H F, et al. Rare helium-bearing compound FeO2He stabilized at deep-Earth conditions [J]. Physical Review Letters, 2018, 121(25): 255703. doi: 10.1103/PhysRevLett.121.255703 [8] SHI J M, CUI W W, HAO J, et al. Formation of ammonia-helium compounds at high pressure [J]. Nature Communications, 2020, 11(1): 3164. doi: 10.1038/s41467-020-16835-z [9] LIU C, GAO H, HERMANN A, et al. Plastic and superionic helium ammonia compounds under high pressure and high temperature [J]. Physical Review X, 2020, 10(2): 021007. doi: 10.1103/PhysRevX.10.021007 [10] LIU C, GAO H, WANG Y, et al. Multiple superionic states in helium-water compounds [J]. Nature Physics, 2019, 15(10): 1065–1070. doi: 10.1038/s41567-019-0568-7 [11] LIU H Y, YAO Y S, KLUG D D. Stable structures of He and H2O at high pressure [J]. Physical Review B, 2015, 91(1): 014102. doi: 10.1103/PhysRevB.91.014102 [12] GAO H, LIU C, HERMANN A, et al. Coexistence of plastic and partially diffusive phases in a helium-methane compound [J]. National Science Review, 2020, 7(10): 1540–1547. doi: 10.1093/nsr/nwaa064 [13] DING S C, ZHANG P, YANG K, et al. Formation of solid SiO2He compound at high pressure and high temperature [J]. Physical Review B, 2022, 106(2): 024102. doi: 10.1103/PhysRevB.106.024102 [14] SHEN G Y, MEI Q, PRAKAPENKA V B, et al. Effect of helium on structure and compression behavior of SiO2 glass [J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(15): 6004–6007. doi: 10.1073/pnas.1102361108 [15] SATO T, FUNAMORI N, YAGI T. Helium penetrates into silica glass and reduces its compressibility [J]. Nature Communications, 2011, 2: 345. doi: 10.1038/ncomms1343 [16] LI D, LIU Y, TIAN F B, et al. High-pressure structures of helium and carbon dioxide from first-principles calculations [J]. Solid State Communications, 2018, 283: 9–13. doi: 10.1016/j.ssc.2018.06.012 [17] MONSERRAT B, MARTINEZ-CANALES M, NEEDS R J, et al. Helium-iron compounds at terapascal pressures [J]. Physical Review Letters, 2018, 121(1): 015301. doi: 10.1103/PhysRevLett.121.015301 [18] TIAN Y F, TSE J S, LIU G T, et al. Predicted crystal structures of xenon and alkali metals under high pressures [J]. Physical Chemistry Chemical Physics, 2022, 24(30): 18119–18123. doi: 10.1039/D2CP02657K [19] ZOU M, YANG K, ZHANG P, et al. Existence of solid Na-Xe compounds at the extreme conditions of Earth’s interior [J]. Physical Review Research, 2023, 5(4): 043107. doi: 10.1103/PhysRevResearch.5.043107 [20] LU C W, YANG K, CUI W W, et al. Stability of XeK4 compound under extreme conditions [J]. Physical Review B, 2024, 110(2): 024111. doi: 10.1103/PhysRevB.110.024111 [21] LIU Z, BOTANA J, MIAO M S, et al. Unexpected Xe anions in XeLin intermetallic compounds [J]. Europhysics Letters, 2017, 117(2): 26002. doi: 10.1209/0295-5075/117/26002 [22] KRESSE G, FURTHMÜLLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set [J]. Physical Review B, 1996, 54(16): 11169–11186. doi: 10.1103/PhysRevB.54.11169 [23] VANDERLEI DOS SANTOS A, PADILHA G, MONÇALVES M. Determination of the stability and magnetic properties of Fe-Pd nitride using the generalised gradient approximation (GGA) [J]. Solid State Sciences, 2012, 14(2): 269–275. doi: 10.1016/j.solidstatesciences.2011.11.031 [24] WANG Y C, LV J, ZHU L, et al. Crystal structure prediction via particle-swarm optimization [J]. Physical Review B, 2010, 82(9): 094116. doi: 10.1103/PhysRevB.82.094116 [25] WANG Y C, LV J, ZHU L, et al. CALYPSO: a method for crystal structure prediction [J]. Computer Physics Communications, 2012, 183(10): 2063–2070. doi: 10.1016/j.cpc.2012.05.008 [26] GAO B, GAO P Y, LU S H, et al. Interface structure prediction via CALYPSO method [J]. Science Bulletin, 2019, 64(5): 301–309. doi: 10.1016/j.scib.2019.02.009 [27] SHAO X C, LV J, LIU P, et al. A symmetry-orientated divide-and-conquer method for crystal structure prediction [J]. The Journal of Chemical Physics, 2022, 156(1): 014105. doi: 10.1063/5.0074677 [28] BLÖCHL P E, JEPSEN O, ANDERSEN O K. Improved tetrahedron method for Brillouin-zone integrations [J]. Physical Review B, 1994, 49(23): 16223–16233. doi: 10.1103/PhysRevB.49.16223 [29] MOMMA K, IZUMI F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data [J]. Journal of Applied Crystallography, 2011, 44(6): 1272–1276. doi: 10.1107/S0021889811038970 [30] LIU Z, BOTANA J, HERMANN A, et al. Reactivity of He with ionic compounds under high pressure [J]. Nature Communications, 2018, 9(1): 951. doi: 10.1038/s41467-018-03284-y [31] HUANG Y S, SONG H X, HAO Q D, et al. He-Mg compounds and helium-driven nonmetal transition in metallic magnesium [J]. Physical Review B, 2024, 110(21): 214102. doi: 10.1103/PhysRevB.110.214102 [32] TANG H Y, LIU J Y, SHA Y X, et al. Pressures-stabilized unconventional stoichiometric electride Mg3S2 [J]. Physical Review B, 2025, 111(18): 184104. doi: 10.1103/PhysRevB.111.184104 -






 下载:
下载: