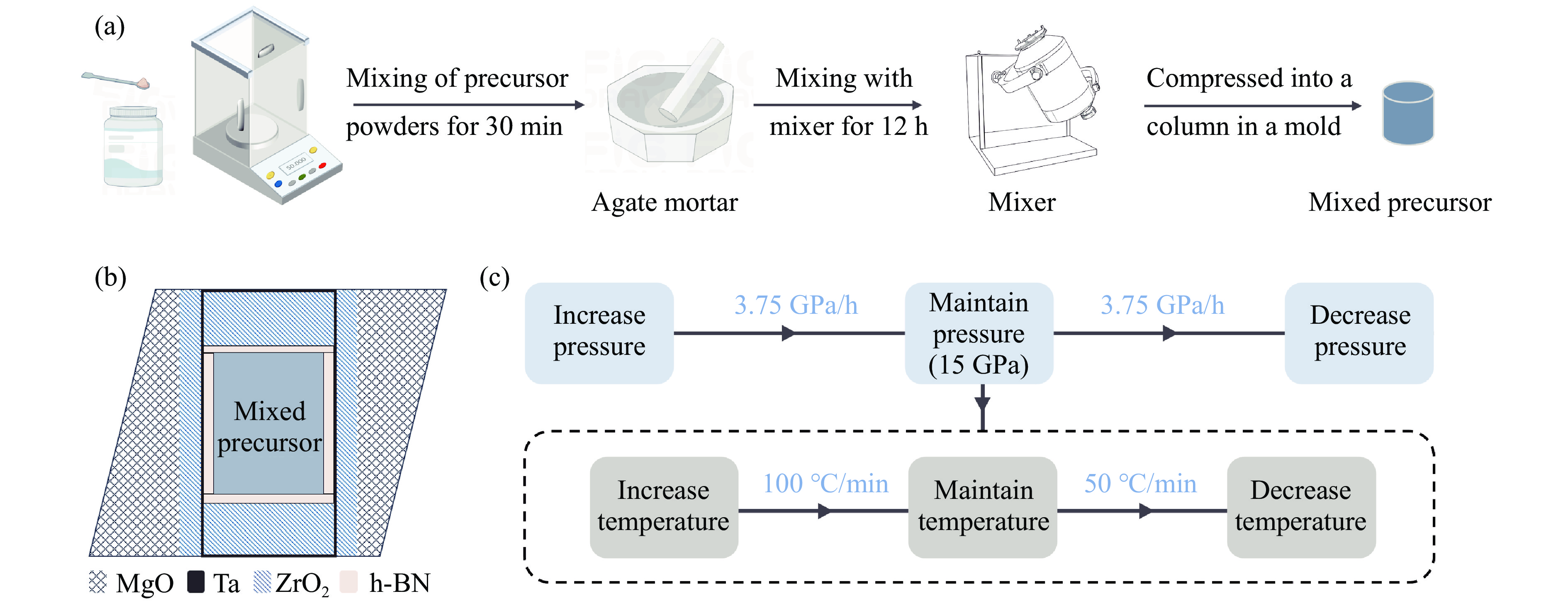
Synthesis of Platinum-Group Metal Nitride OsNx through High-Pressure Coupling Reaction
-
摘要: 铂族金属氮化物是一类新型超不可压缩超硬材料,通常借助激光加热金刚石压砧(laser-heated diamond anvil cell,LHDAC)技术,通过单质元素化合反应法(A+B=AB)在高温高压下(
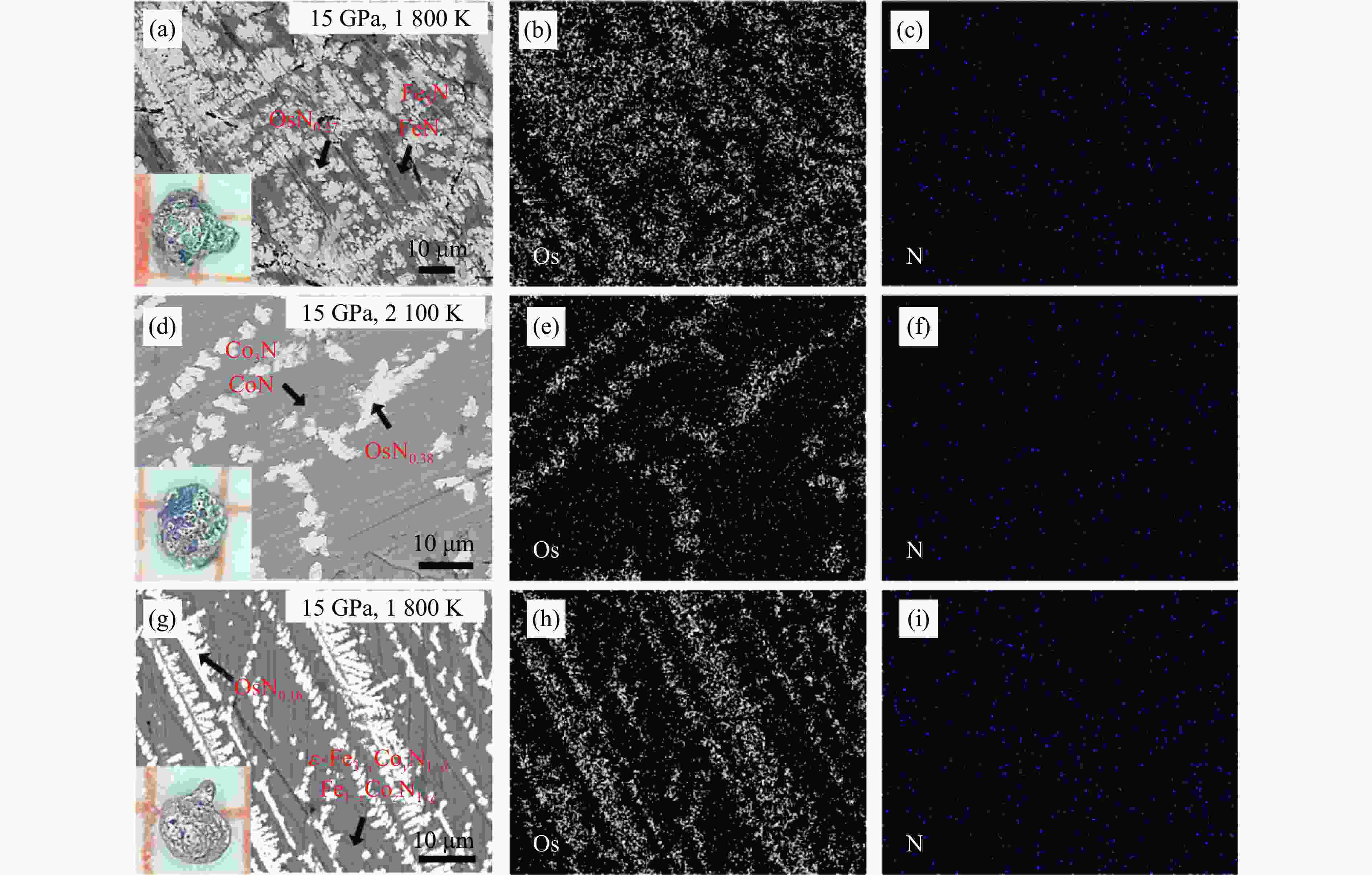
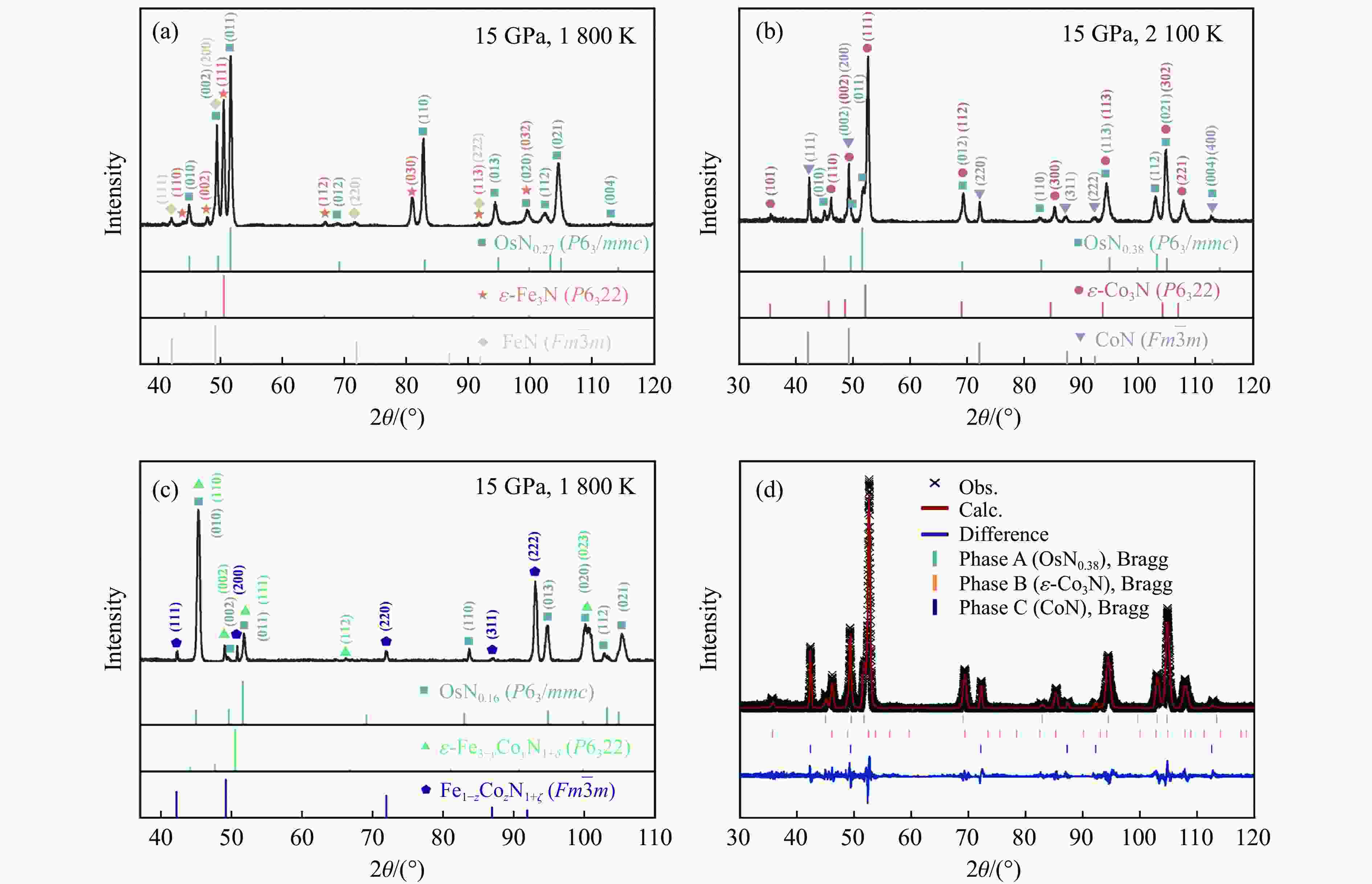
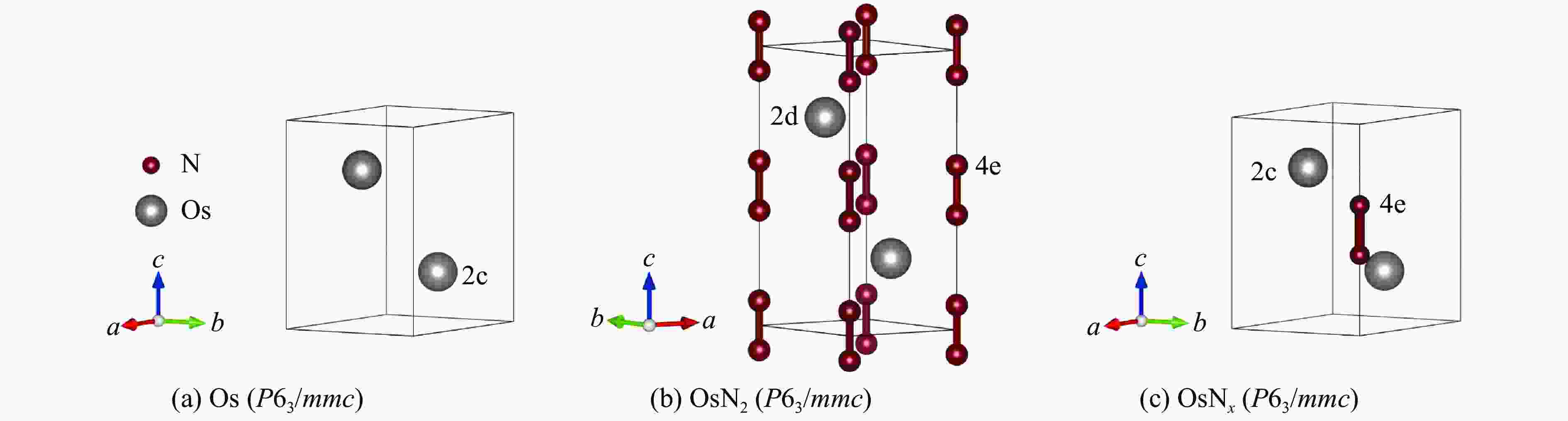
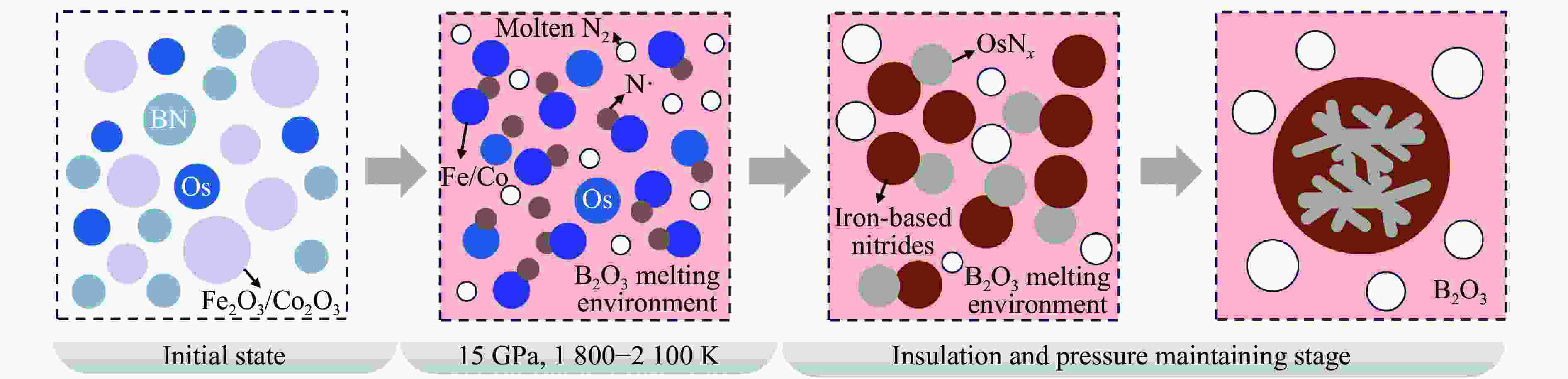
2000 K、高于45 GPa)合成,探索有效降低合成压力的非常规化学合成方法对于开发和利用铂族金属(platinum-group metals,PGM)氮化物具有重要意义。以Fe2O3/Co2O3、h-BN、Os粉为反应前驱体,在大腔体压机提供的高温高压(1800 ~2100 K、15 GPa)条件下,首次通过新型高压偶联(high-pressure coupling,HPC)反应合成了OsNx(0.16≤x≤0.38)。HPC反应合成出的金属块状产物一般为OsNx与铁基氮化物复合的块体合金,利用微区X射线衍射、扫描电子显微镜对块状合金产物进行物相和结构表征,结果显示,HPC反应可以在远低于高压单质元素化合反应所需50 GPa压力阈值的条件下合成理论预测的六方OsN2结构的OsNx(空间群为P63/mmc),N原子在Os晶体内部分占据晶格的间隙位。HPC反应能够有效降低金属Os氮化的能量势垒,形成非化学计量比的OsNx化合物,为在低压条件下制备铂族金属氮化物块体材料开辟了一条新的合成途径。Abstract: Platinum-group metals (PGMs) nitrides represent a new class of super incompressible superhard materials, typically synthesized under extreme conditions (above 45 GPa,2000 K) using laser-heated diamond anvil cell (LHDAC) technology via monatomic elemental chemosynthesis (A+B=AB). Exploring non-conventional synthesis methods that significantly reduce the required pressures is crucial for advancing the development and application of PGMs nitrides. In this work, OsNx (0.16≤x≤0.38) was synthesized for the first time via a novel high-pressure coupling (HPC) reaction, using Fe2O3/Co2O3, h-BN, and Os powders as precursors under high-temperature and high-pressure conditions (15 GPa,1800 −2100 K) in a large-volume press. The HPC-synthesized metal bulk products primarily consist of OsNx alloyed with iron-based nitrides. Phase composition and structural characterization via X-ray powder diffraction (XRD) and scanning electron microscope (SEM) confirm the formation of hexagonal OsN2 (space group P63/mmc), as theoretically predicted, at pressures well below the 50 GPa threshold typically required for high-pressure monatomic elemental combination reactions. The nitrogen atoms partially occupy interstitial sites within the Os crystal structure. This study demonstrates that the HPC reaction effectively lowers the energy barrier for Os nitration, facilitating the formation of non-stoichiometric OsNx compounds. These findings open a new synthetic route for bulk PGM nitride materials under significantly reduced pressure conditions. -
表 1 典型实验的前驱体、温度压力条件及块体合金产物的能谱元素分析结果
Table 1. Precursors, temperature-pressure conditions, and EDX analysis results of bulk alloy products in typical experiments
Exp. No. Precursors p/GPa T/K Atom fraction in the light area/% Atom fraction in the gray area/% Os N Fe Co N 1 3Fe2O3, 6BN, Os 15 1800 78.61 21.39 65.49 34.61 2 3Co2O3, 6BN, Os 15 2100 72.41 27.59 74.71 25.29 3 3Fe2O3, 3Co2O3, 12BN, Os 15 1800 77.34 12.66 26.81 45.81 27.38 表 2 Os、OsN2和OsN0.38的晶胞参数对比
Table 2. Comparison of unit cell parameters for Os, OsN2, and OsN0.38
-
[1] WEILAND R, LUPTON D F, FISCHER B, et al. High-temperature mechanical properties of the platinum group metals: properties of pure iridium at high temperature [J]. Platinum Metals Review, 2006, 50(4): 158–170. doi: 10.1595/147106706X154198 [2] GUNN G. Platinum-group metals [M]//GUNN G. Critical Metals Handbook. Hoboken: Wiley, 2014: 284–311. [3] IVANOVSKII A L. Platinum group metal nitrides and carbides: synthesis, properties and simulation [J]. Russian Chemical Reviews, 2009, 78(4): 303–318. doi: 10.1070/RC2009v078n04ABEH004036 [4] CHEN W, TSE J S, JIANG J Z. An ab initio study of 5d noble metal nitrides: OsN2, IrN2, PtN2 and AuN2 [J]. Solid State Communications, 2010, 150(3/4): 181–186. doi: 10.1016/j.ssc.2009.10.029 [5] YU R, ZHAN Q, ZHANG X F. Elastic stability and electronic structure of pyrite type PtN2: a hard semiconductor [J]. Applied Physics Letters, 2006, 88(5): 051913. doi: 10.1063/1.2168683 [6] YOUNG A F, SANLOUP C, GREGORYANZ E, et al. Synthesis of novel transition metal nitrides IrN2 and OsN2 [J]. Physical Review Letters, 2006, 96(15): 155501. doi: 10.1103/PhysRevLett.96.155501 [7] GREGORYANZ E, SANLOUP C, SOMAYAZULU M, et al. Synthesis and characterization of a binary noble metal nitride [J]. Nature Materials, 2004, 3(5): 294–297. doi: 10.1038/nmat1115 [8] FU H Z, LIU W F, PENG F, et al. Theoretical investigations of structural, elastic and thermodynamic properties for PtN2 under high pressure [J]. Physica B: Condensed Matter, 2009, 404(1): 41–46. doi: 10.1016/j.physb.2008.10.001 [9] CROWHURST J C, GONCHAROV A F, SADIGH B, et al. Synthesis and characterization of the nitrides of platinum and iridium [J]. Science, 2006, 311(5765): 1275–1278. doi: 10.1126/science.1121813 [10] CHEN Z W, GUO X J, LIU Z Y, et al. Crystal structure and physical properties of OsN2 and PtN2 in the marcasite phase [J]. Physical Review B, 2007, 75(5): 054103. doi: 10.1103/physrevb.75.054103 [11] LI Y W, MA Y M. Crystal structure and physical properties of OsN: first-principle calculations [J]. Solid State Communications, 2010, 150(15/16): 759–762. doi: 10.1016/j.ssc.2010.01.026 [12] ZHAO W J, XU H B, WANG Y X. A hard semiconductor OsN4 with high elastic constant c44 [J]. Physica Status Solidi (RRL)–Rapid Research Letters, 2009, 3(7/8): 272–274. doi: 10.1002/pssr.200903252 [13] ZHENG J C. Superhard hexagonal transition metal and its carbide and nitride: Os, OsC, and OsN [J]. Physical Review B, 2005, 72(5): 052105. doi: 10.1103/PhysRevB.72.052105 [14] SOTO G. Computational study of Hf, Ta, W, Re, Ir, Os and Pt pernitrides [J]. Computational Materials Science, 2012, 61: 1–5. doi: 10.1016/j.commatsci.2012.03.056 [15] LEI L, HE D W. Synthesis of GaN crystals through solid-state metathesis reaction under high pressure [J]. Crystal Growth & Design, 2009, 9(3): 1264–1266. doi: 10.1021/cg801017h [16] LEI L, ZHANG L L. Recent advance in high-pressure solid-state metathesis reactions [J]. Matter and Radiation at Extremes, 2018, 3(3): 95–103. doi: 10.1016/j.mre.2017.12.003 [17] 高上攀, 雷力, 胡启威, 等. 三元铁基金属氮化物的高压复分解反应合成 [J]. 高压物理学报, 2016, 30(4): 265–270. doi: 10.11858/gywlxb.2016.04.001GAO S P, LEI L, HU Q W, et al. High-pressure solid-state metathesis synthesis of ternary iron-based metal nitrides [J]. Chinese Journal of High Pressure Physics, 2016, 30(4): 265–270. doi: 10.11858/gywlxb.2016.04.001 [18] LEI L, YIN W W, JIANG X D, et al. Synthetic route to metal nitrides: high-pressure solid-state metathesis reaction [J]. Inorganic Chemistry, 2013, 52(23): 13356–13362. doi: 10.1021/ic4014834 [19] ZHANG H Y, WU B B, LIU J Y, et al. High-pressure coupling reactions to produce a spherical bulk RexN/Fe3N composite [J]. Inorganic Chemistry, 2023, 62(16): 6263–6273. doi: 10.1021/acs.inorgchem.2c04089 [20] OHFUJI H, YAMAMOTO M. EDS quantification of light elements using osmium surface coating [J]. Journal of Mineralogical and Petrological Sciences, 2015, 110(4): 189–195. doi: 10.2465/jmps.141126 [21] GODWAL B K, YAN J, CLARK S M, et al. High-pressure behavior of osmium: an analog for iron in Earth’s core [J]. Journal of Applied Physics, 2012, 111(11): 112608. doi: 10.1063/1.4726203 [22] WU B B, ZHANG F, HU Q W, et al. The effect of interstitial-site nitrogen on structural, elastic, and magnetic properties of face-center cubic Co [J]. Journal of Applied Physics, 2021, 129(10): 105901. doi: 10.1063/5.0037917 [23] PATEL N N, SUNDER M. High pressure melting curve of osmium up to 35 GPa [J]. Journal of Applied Physics, 2019, 125(5): 055902. doi: 10.1063/1.5045823 -






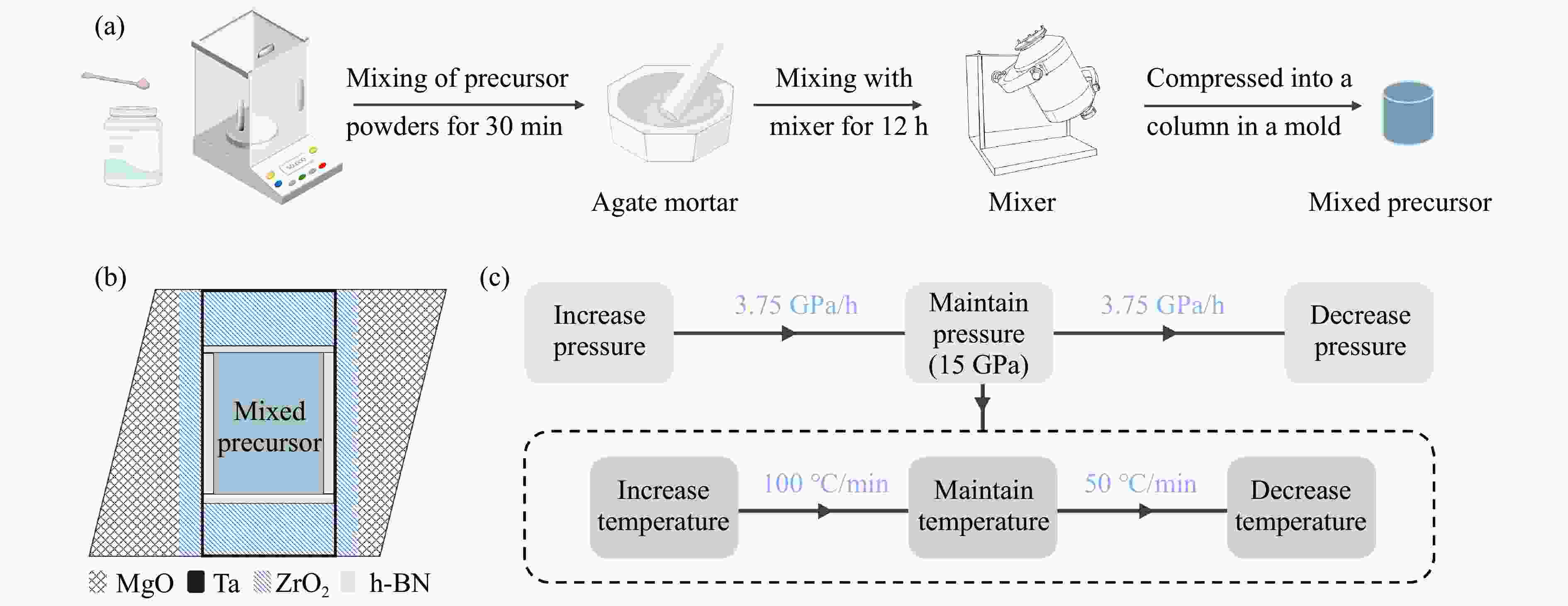
 下载:
下载: