Effect of Metal Oxides on the Combustion Characteristics of Al-Based Thermite
-
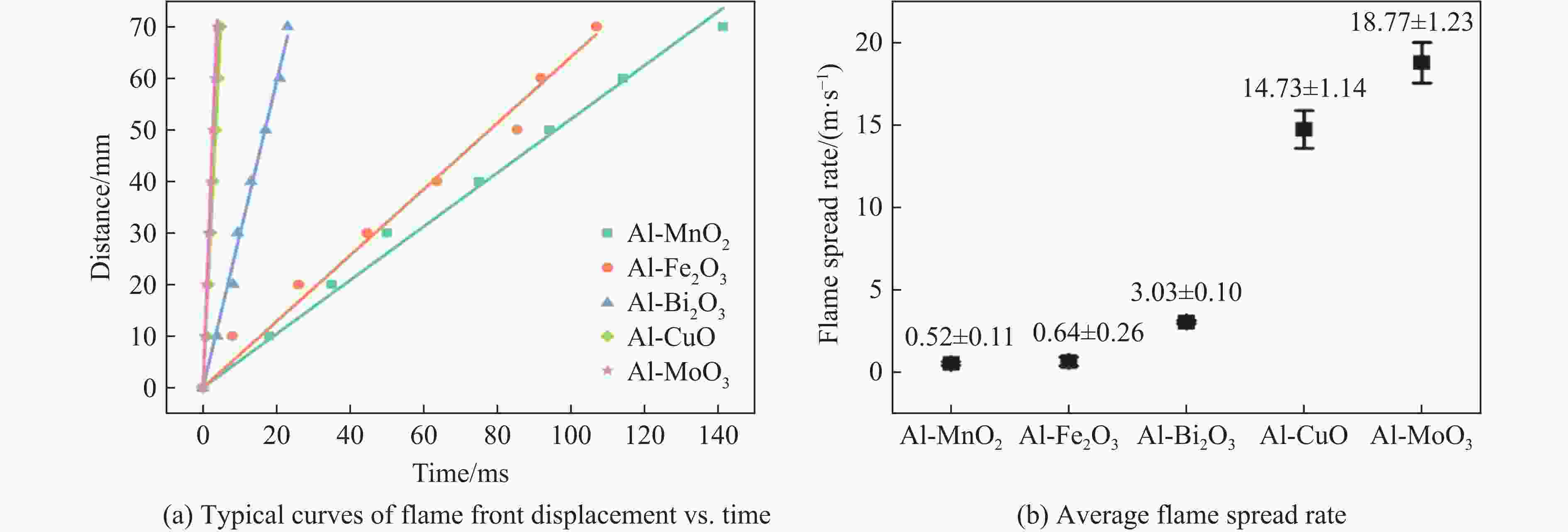
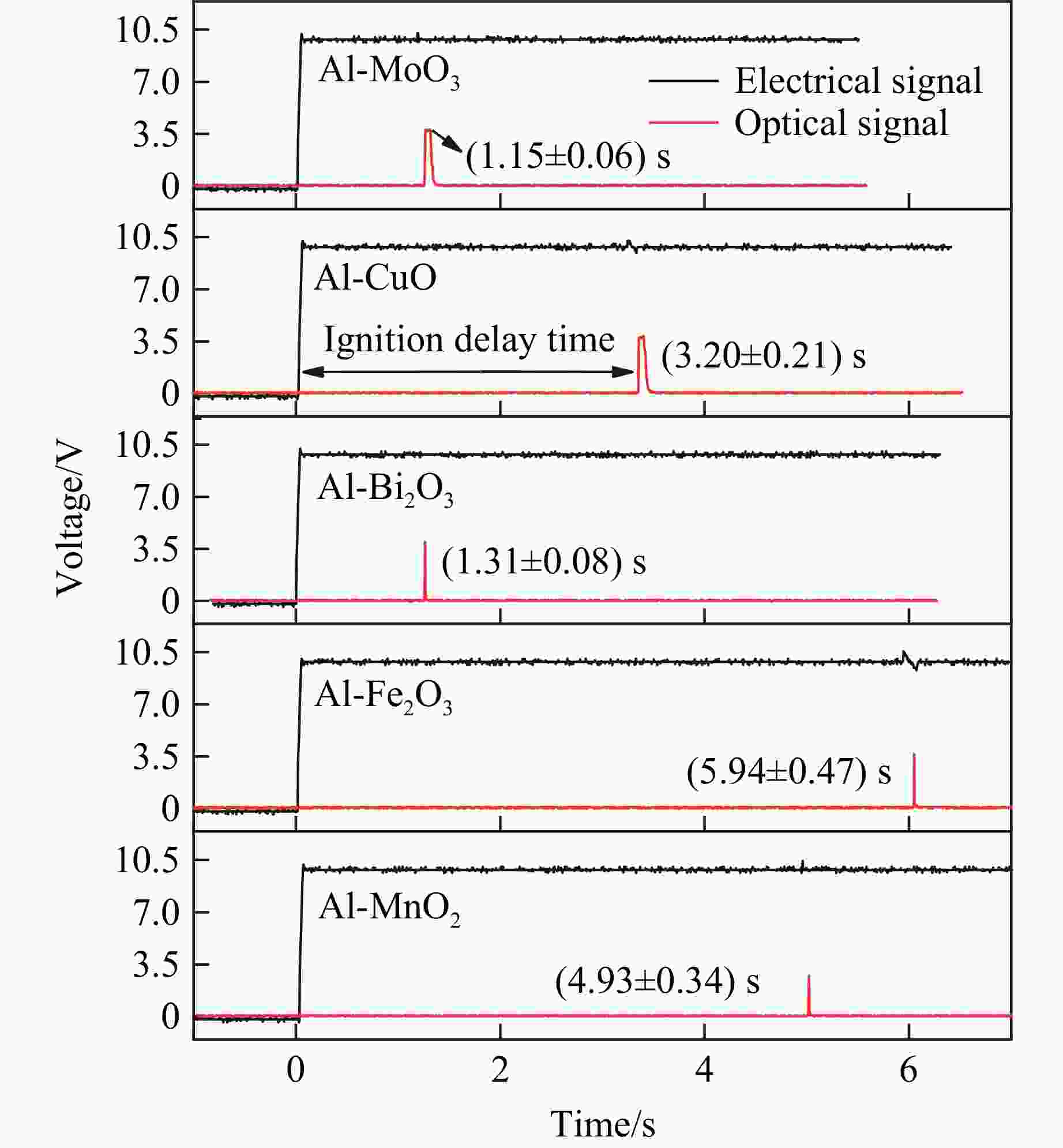
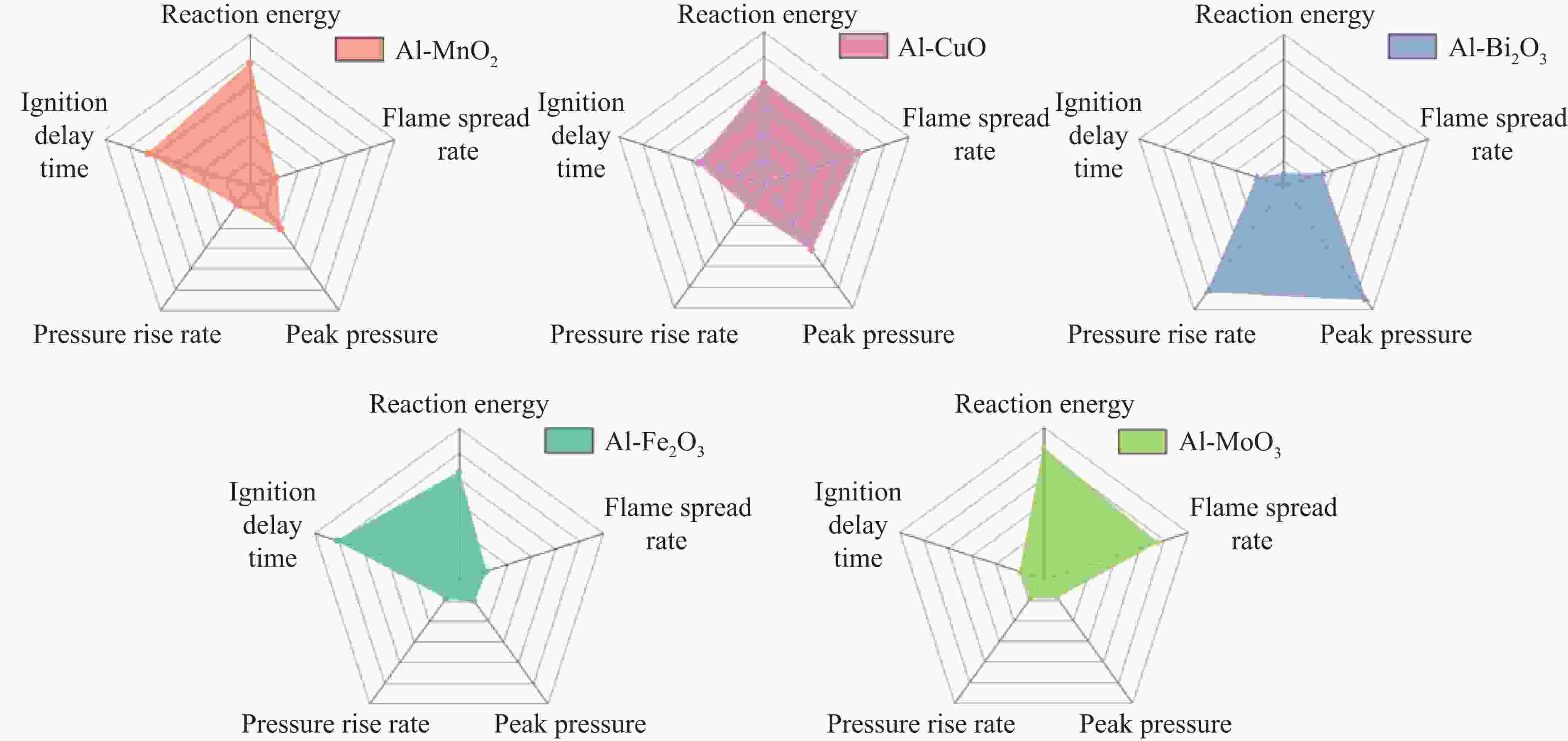
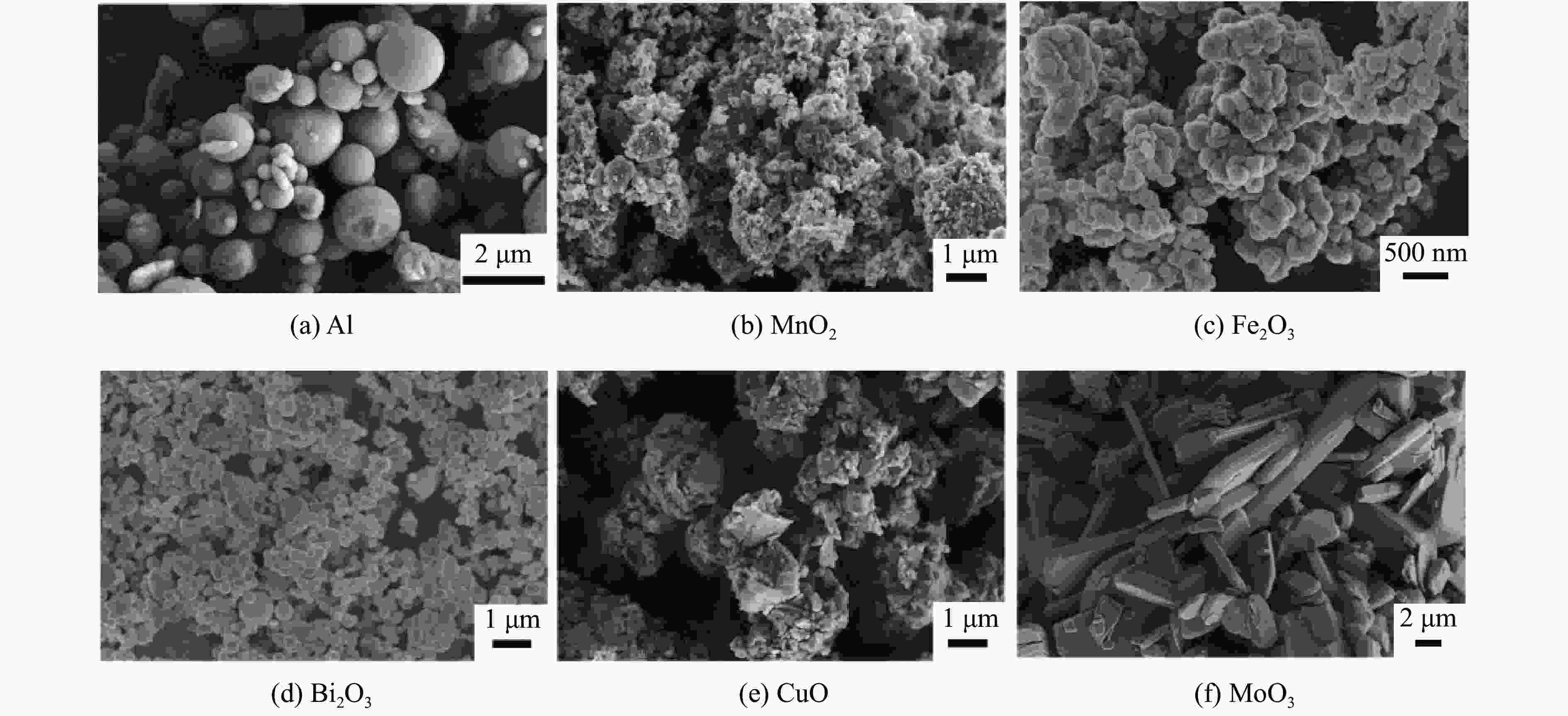
摘要: 为了探究金属氧化物种类对铝热剂燃烧特性的影响,选取了Bi2O3、Fe2O3、MnO2、CuO和MoO3 5种金属氧化物,采用液相混合制备了二元铝热剂,对5种铝热剂的反应热值、自蔓延燃烧特性、反应压力与点火延迟时间等燃烧特性进行了系统研究。结果表明:金属氧化物种类对铝热剂的燃烧特性有显著影响;Al-MoO3具有最高反应热(氩气中为(4.10±0.05) kJ/g)、火焰蔓延速率((18.77±1.23) m/s)、火焰温度以及最短的点火延迟时间((1.15±0.06) s);Al-Bi2O3表现出最高的压力峰值和升压速率,压力峰值分别为Al-CuO、Al-MnO2、Al-Fe2O3、Al-MoO3的1.9、3.5、14.6、24.3倍。通过选择合适的金属氧化剂,可以实现铝热剂燃烧特性调控,为其在军事和工业领域应用提供参考。Abstract: To explore the influence of various metal oxides on the combustion behavior of Al-based thermites, five metal oxides (Bi2O3, Fe2O3, MnO2, CuO, and MoO3), were selected and synthesized via a liquid-phase mixing method. The combustion characteristics, including reaction energy, self-propagating combustion properties, reaction pressure, and ignition delay time, were systematically investigated. The results demonstrated that the choice of metal oxide significantly impacted the combustion performance of Al-based thermites. Among them, Al-MoO3 exhibited the highest reaction energy in an Ar atmosphere ((4.10±0.05) kJ/g), the fastest flame spead rate ((18.77±1.23) m/s), the highest flame temperature, and the shortest ignition delay time ((1.15±0.06) s). Meanwhile, Al-Bi2O3 generated the highest peak pressure and pressure rise rate, with its peak pressure being 1.9, 3.5, 14.6, and 24.3 times greater than those of Al-CuO, Al-MnO2, Al-Fe2O3, and Al-MoO3, respectively. These findings highlight the potential to regulate thermite combustion properties through strategic metal oxide selection, providing a theoretical foundation for military and industrial applications.
-
Key words:
- Al thermite /
- combustion properties /
- metal oxides /
- reaction pressure
-
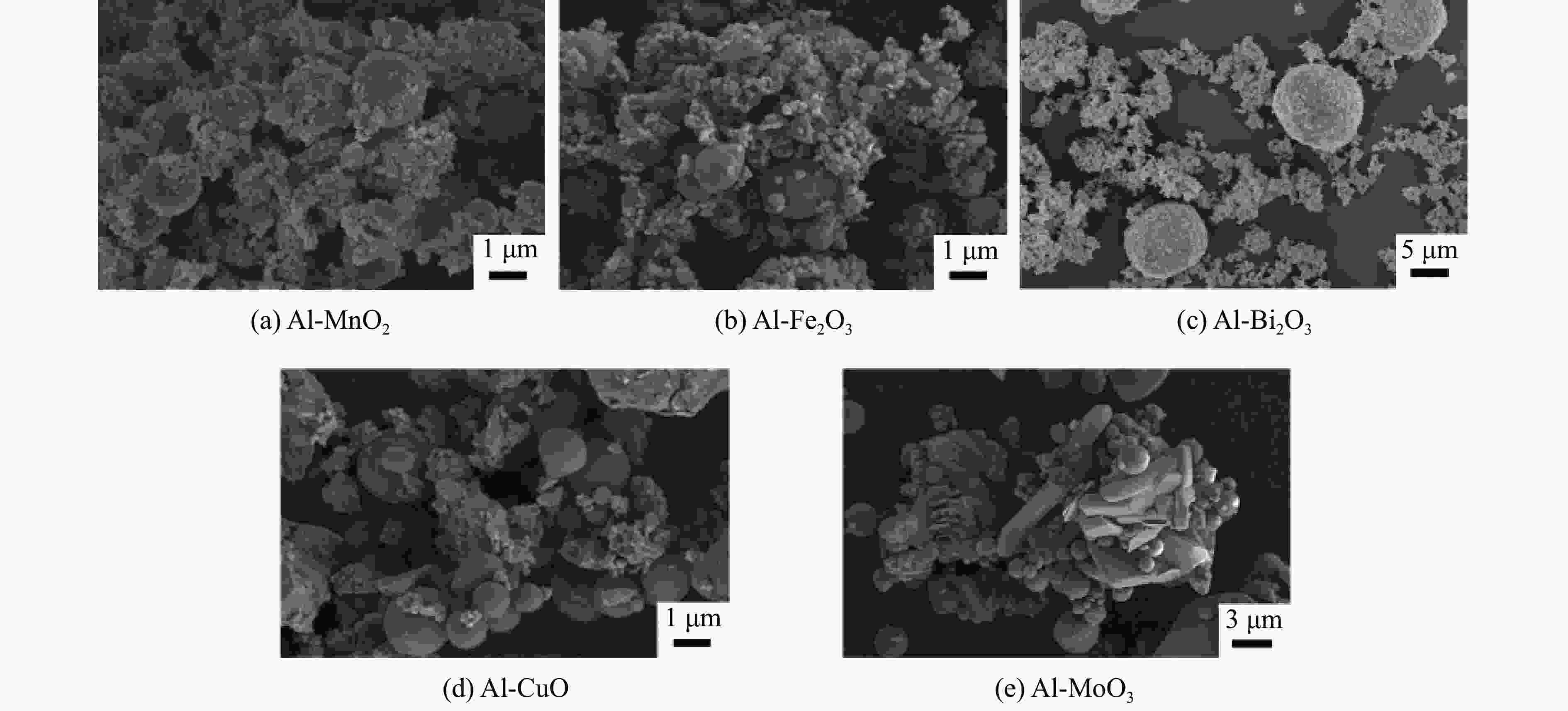
表 1 铝热剂的成分配比
Table 1. Composition ratio of different Al-based thermites
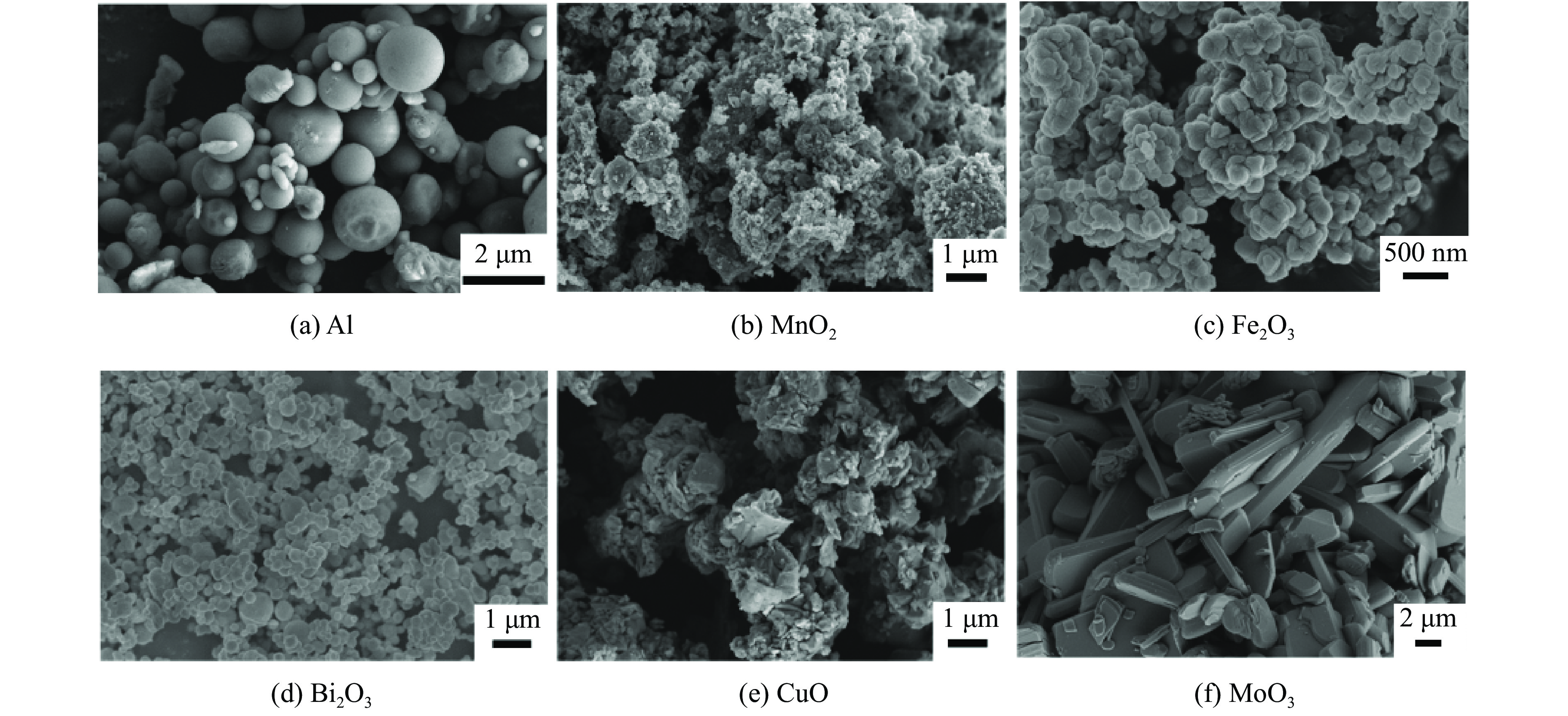
Thermites Mass fraction of Al/% Mass fraction of metal oxides/% Al-MnO2 29 71 Al-Fe2O3 25 75 Al-Bi2O3 10 90 Al-CuO 18 82 Al-MoO3 27 73 表 2 5种铝热剂的反应热及反应效率
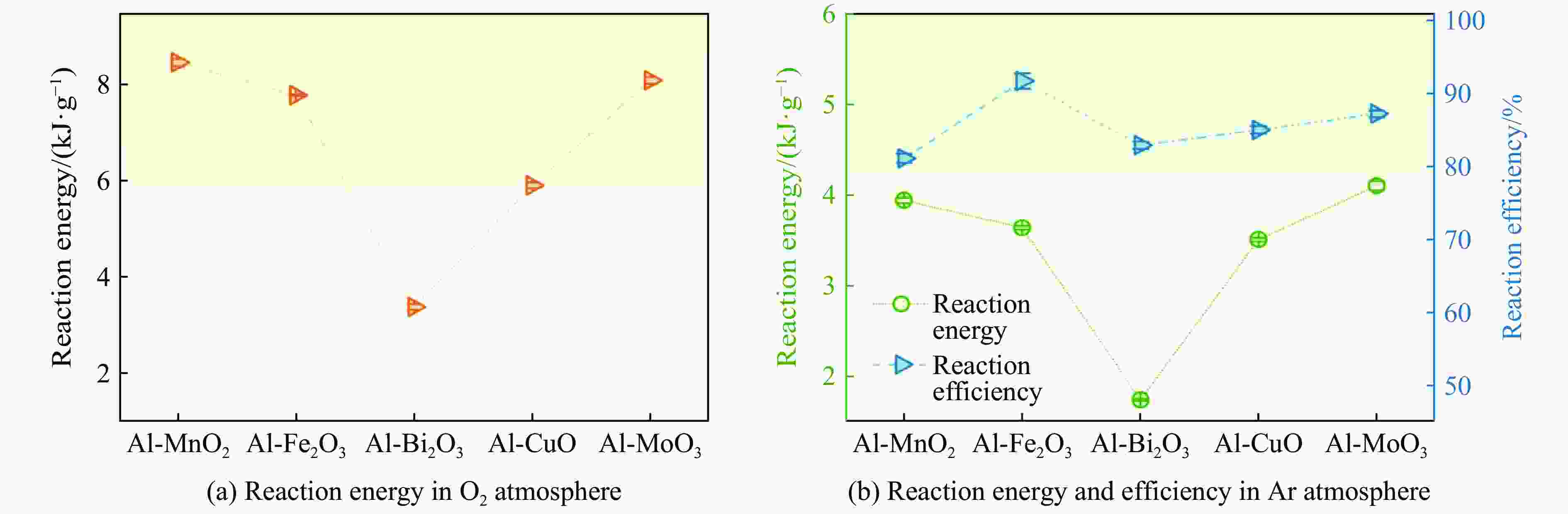
Table 2. Reaction energy and efficiencies of five Al-based thermites
Thermites Reaction energy in Ar Reaction energy in O2/(kJ·g–1) Theoretical/(kJ·g–1) Measured/(kJ·g–1) Efficiency/% Al-MnO2 4.86 3.94±0.03 81.10±0.62 8.46±0.08 Al-Fe2O3 3.97 3.64±0.02 91.70±1.06 7.78±0.02 Al-Bi2O3 2.11 1.75±0.04 82.90±0.48 3.39±0.06 Al-CuO 4.13 3.51±0.02 85.00±0.51 5.91±0.06 Al-MoO3 4.70 4.10±0.05 87.20±0.47 8.09±0.07 表 3 5种铝热剂的反应压力特性和产物金属的沸点
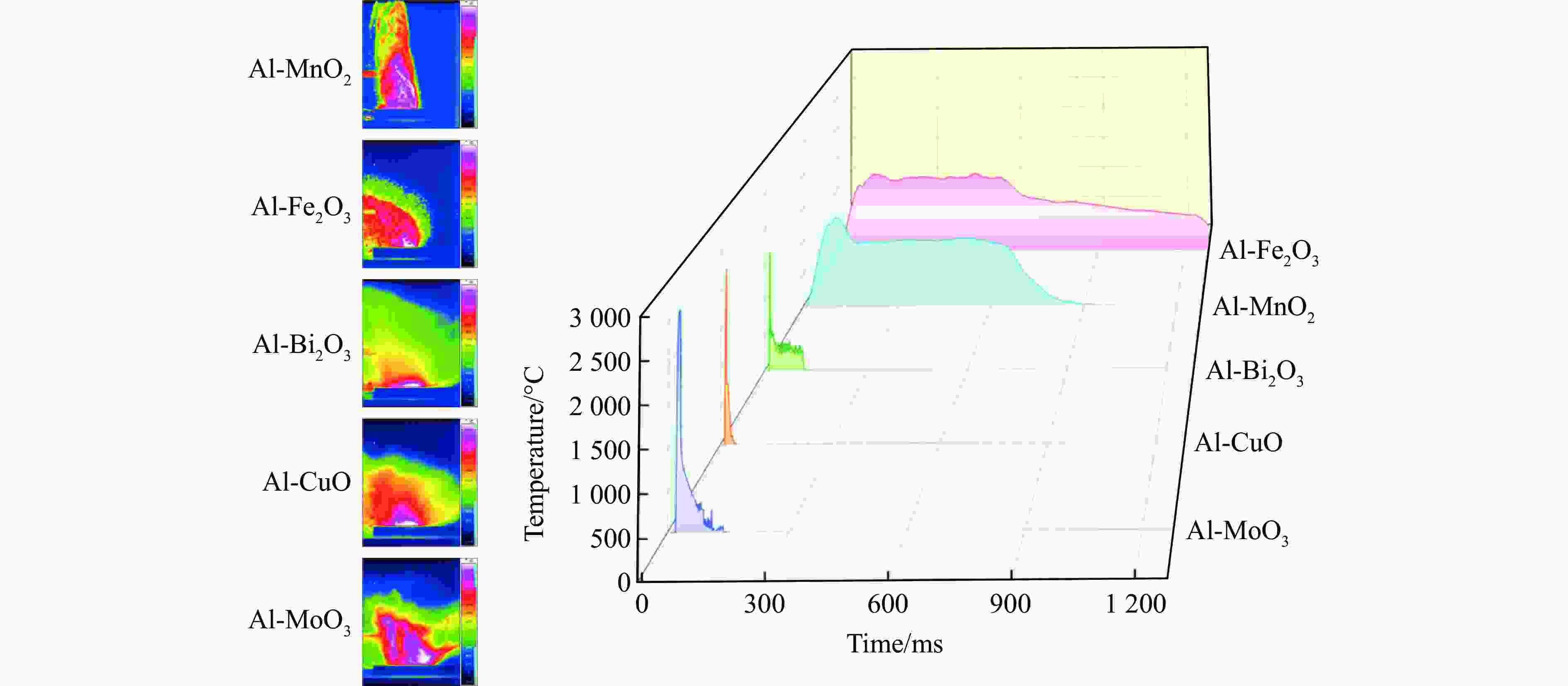
Table 3. Pressure characteristics and boiling points of product metals of five Al-based thermites
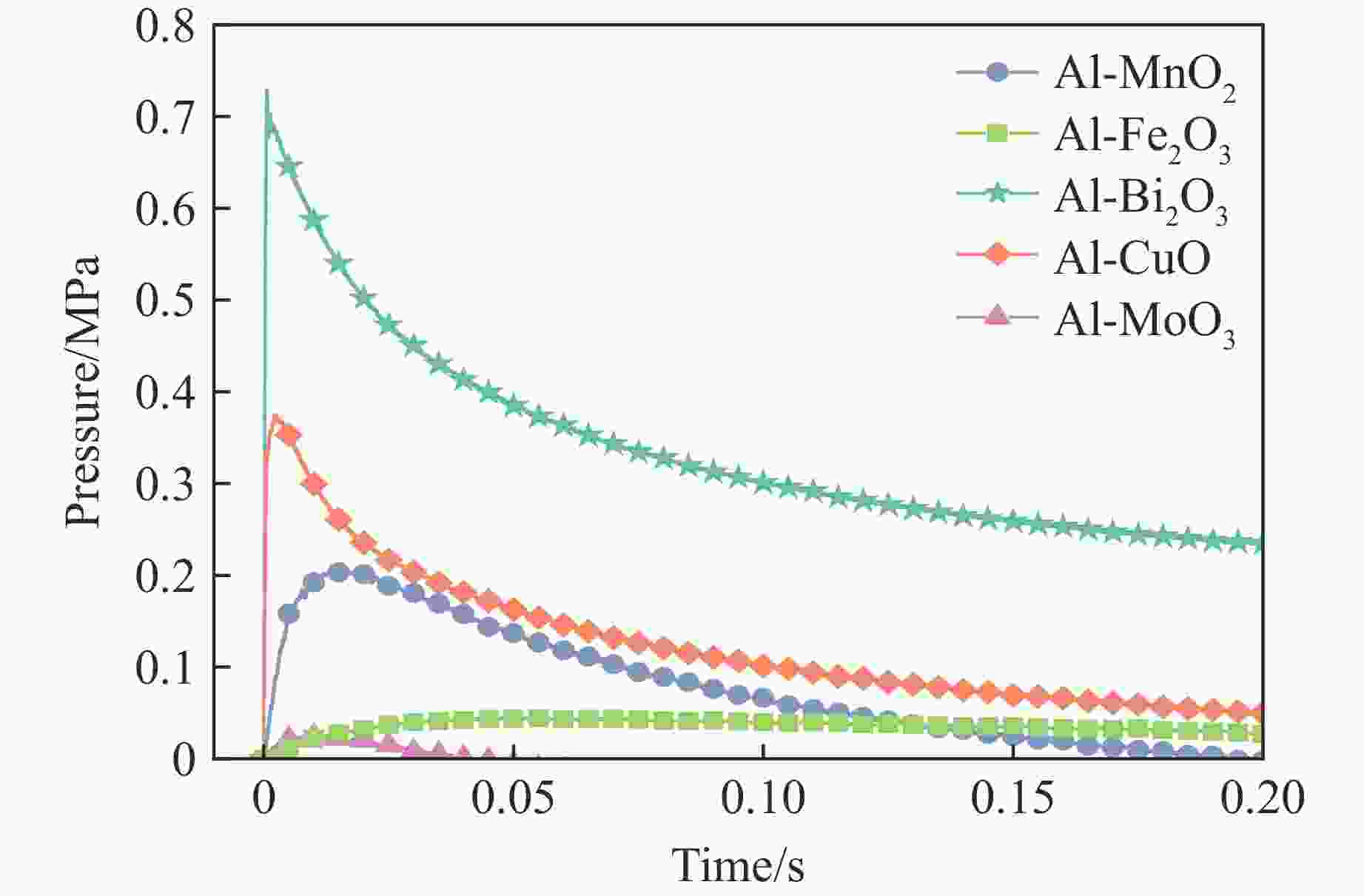
Thermites Peak pressure/MPa Pressure rise rate/(MPa·s–1) Product metals Boiling point of product/℃ Al-MnO2 0.21±0.04 14.0±3.0 Mn 2061 Al-Fe2O3 0.05±0.01 1.0±0.3 Fe 2750 Al-Bi2O3 0.73±0.07 2486.0 ±244.0Bi 1564 Al-CuO 0.38±0.06 141.0±21.0 Cu 2562 Al-MoO3 0.03±0.01 4.0±1.5 Mo 4639 -
[1] HE W, LIU P J, HE G Q, et al. Highly reactive metastable intermixed composites (MICs): preparation and characterization [J]. Advanced Materials, 2018, 30(41): 1706293. doi: 10.1002/adma.201706293 [2] ZHANG S, LIU J X, YANG M, et al. Effects of multi-component co-addition on reaction characteristics and impact damage properties of reactive material [J]. Materials & Design, 2018, 153: 1–8. doi: 10.1016/j.matdes.2018.04.077 [3] CHEN J L, GUO T, SONG J X, et al. The characteristics of combustion reactions involving thermite under different shell materials [J]. RSC Advances, 2020, 10(56): 33762–33769. doi: 10.1039/D0RA05415A [4] BRATTON K R, HILL K J, WOODRUFF C, et al. Tailoring impact debris dispersion using intact or fragmented thermite projectiles [J]. Journal of Applied Physics, 2020, 128(15): 155108. doi: 10.1063/5.0023990 [5] JOSEFSON B L, BISSCHOP R, MESSAADI M, et al. Residual stresses in thermite welded rails: significance of additional forging [J]. Welding in the World, 2020, 64(7): 1195–1212. doi: 10.1007/s40194-020-00912-4 [6] MA X X, LI Y X, HUSSAIN I, et al. Core-shell structured nanoenergetic materials: preparation and fundamental properties [J]. Advanced Materials, 2020, 32(30): 2001291. doi: 10.1002/adma.202001291 [7] DENG S L, JIANG Y, HUANG S D, et al. Tuning the morphological, ignition and combustion properties of micron-Al/CuO thermites through different synthesis approaches [J]. Combustion and Flame, 2018, 195: 303–310. doi: 10.1016/j.combustflame.2018.04.028 [8] GLAVIER L, TATON G, DUCÉRÉ J M, et al. Nanoenergetics as pressure generator for nontoxic impact primers: comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE [J]. Combustion and Flame, 2015, 162(5): 1813–1820. doi: 10.1016/j.combustflame.2014.12.002 [9] SHIN M S, KIM J K, KIM J W, et al. Reaction characteristics of Al/Fe2O3 nanocomposites [J]. Journal of Industrial and Engineering Chemistry, 2012, 18(5): 1768–1773. doi: 10.1016/j.jiec.2012.04.003 [10] 陈嘉琳, 郭涛, 姚淼, 等. 含不同形貌MoO3的Al/MoO3铝热剂的热性能和燃烧性能 [J]. 含能材料, 2022, 30(2): 121–129. doi: 10.11943/CJEM2021105CHEN J L, GUO T, YAO M, et al. Thermal properties and combustion properties of Al/MoO3 thermite containing MoO3 with different morphologies [J]. Chinese Journal of Energetic Materials, 2022, 30(2): 121–129. doi: 10.11943/CJEM2021105 [11] MARTIROSYAN K S, WANG L, VICENT A, et al. Synthesis and performance of bismuth trioxide nanoparticles for high energy gas generator use [J]. Nanotechnology, 2009, 20(40): 405609. doi: 10.1088/0957-4484/20/40/405609 [12] HE W, LYU J Y, TANG D Y, et al. Control the combustion behavior of solid propellants by using core-shell Al-based composites [J]. Combustion and Flame, 2020, 221: 441–452. doi: 10.1016/j.combustflame.2020.07.006 [13] ZHU Z Y, MA B, TANG C M, et al. Molecular dynamic simulation of thermite reaction of Al nanosphere/Fe2O3 nanotube [J]. Physics Letters A, 2016, 380(1/2): 194–199. doi: 10.1016/j.physleta.2015.09.041 [14] SONG J X, GUO T, YAO M, et al. A comparative study of thermal kinetics and combustion performance of Al/CuO, Al/Fe2O3 and Al/MnO2 nanothermites [J]. Vacuum, 2020, 176: 109339. doi: 10.1016/j.vacuum.2020.109339 [15] ZHUANG Z H, XU K D, LIU B Z, et al. Improved reactivity and energy release performance of core-shell structured fuel-rich Si/PTFE energetic composites [J]. Combustion and Flame, 2023, 255: 112889. doi: 10.1016/j.combustflame.2023.112889 [16] LIU Z H, HE C, ZHUANG Z H, et al. Ignition and energy release performance of dual-oxidant Al/MnO2/CuO ternary thermites under rapid heating conditions [J]. Propellants, Explosives, Pyrotechnics, 2024, 49(7): e202400005. doi: 10.1002/prep.202400005 [17] YANG C, WANG W Y, LI Y H, et al. Quantitative study on chemical effects of actual/simulated recirculated exhaust gases on ignition delay times of n-heptane/ethanol fuel blends at elevated temperature [J]. Fuel, 2020, 263: 116327. doi: 10.1016/j.fuel.2019.116327 -






 下载:
下载: