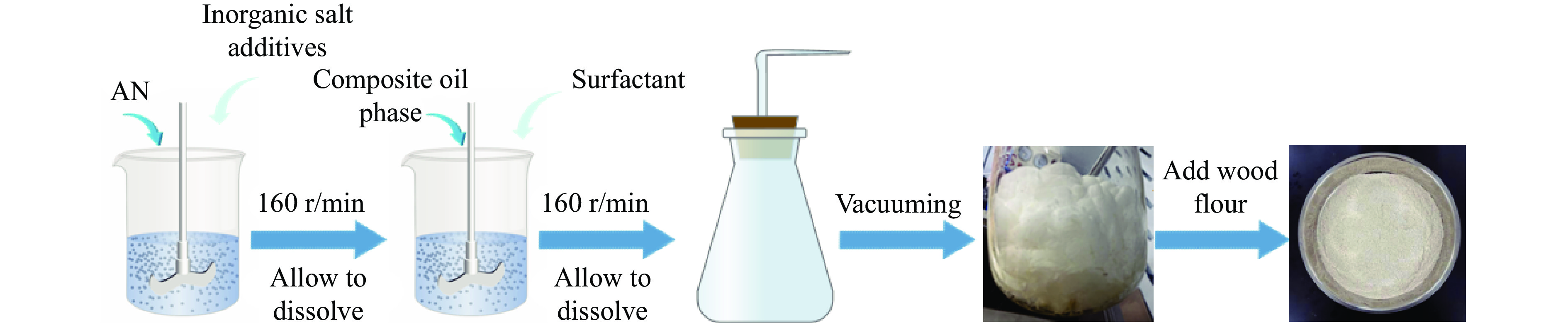
Influence of Inorganic Salts on the Dissolution Temperature of Ammonium Nitrate and the Explosive Performance of Expanded Ammonium Nitrate Explosives
-
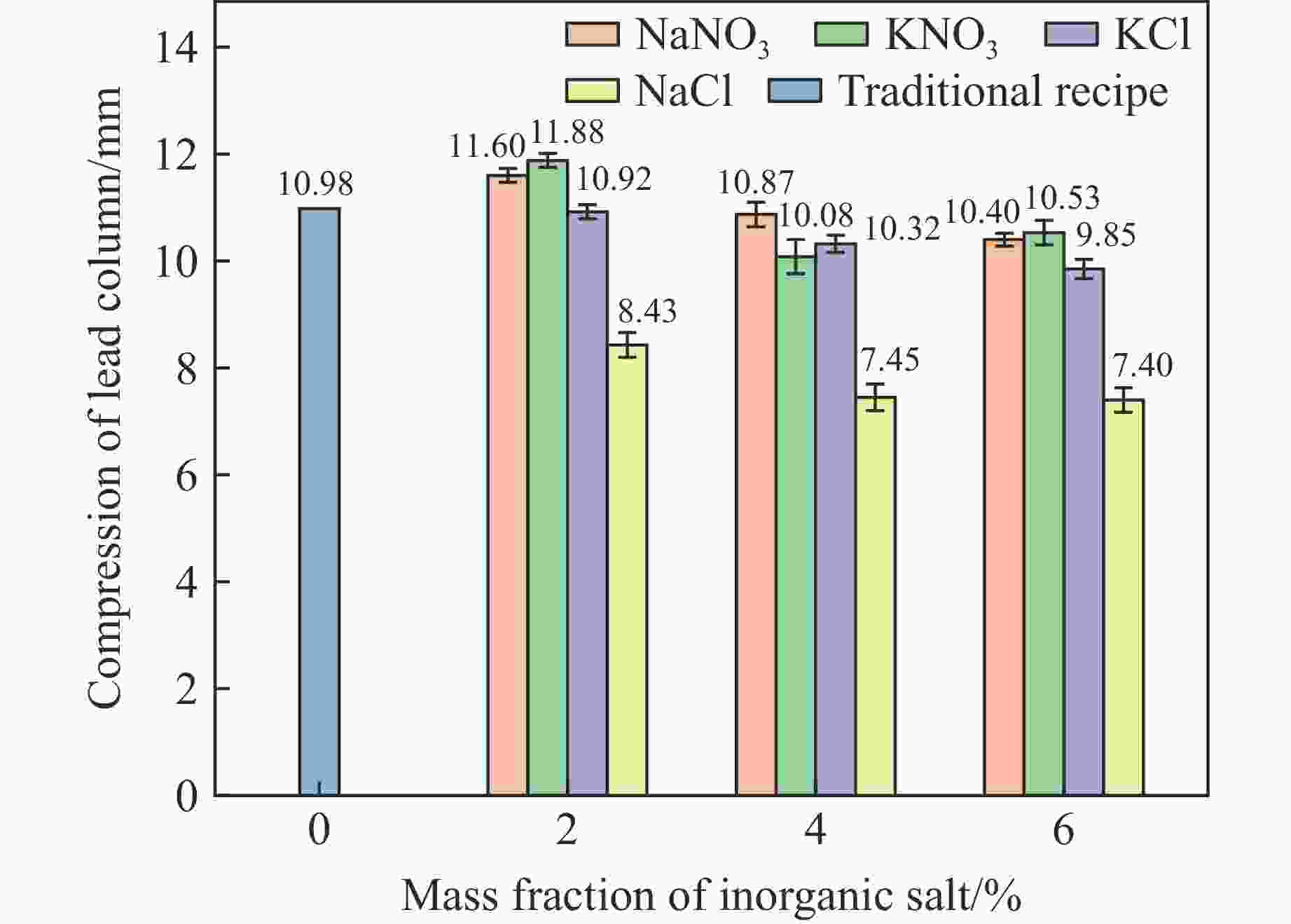
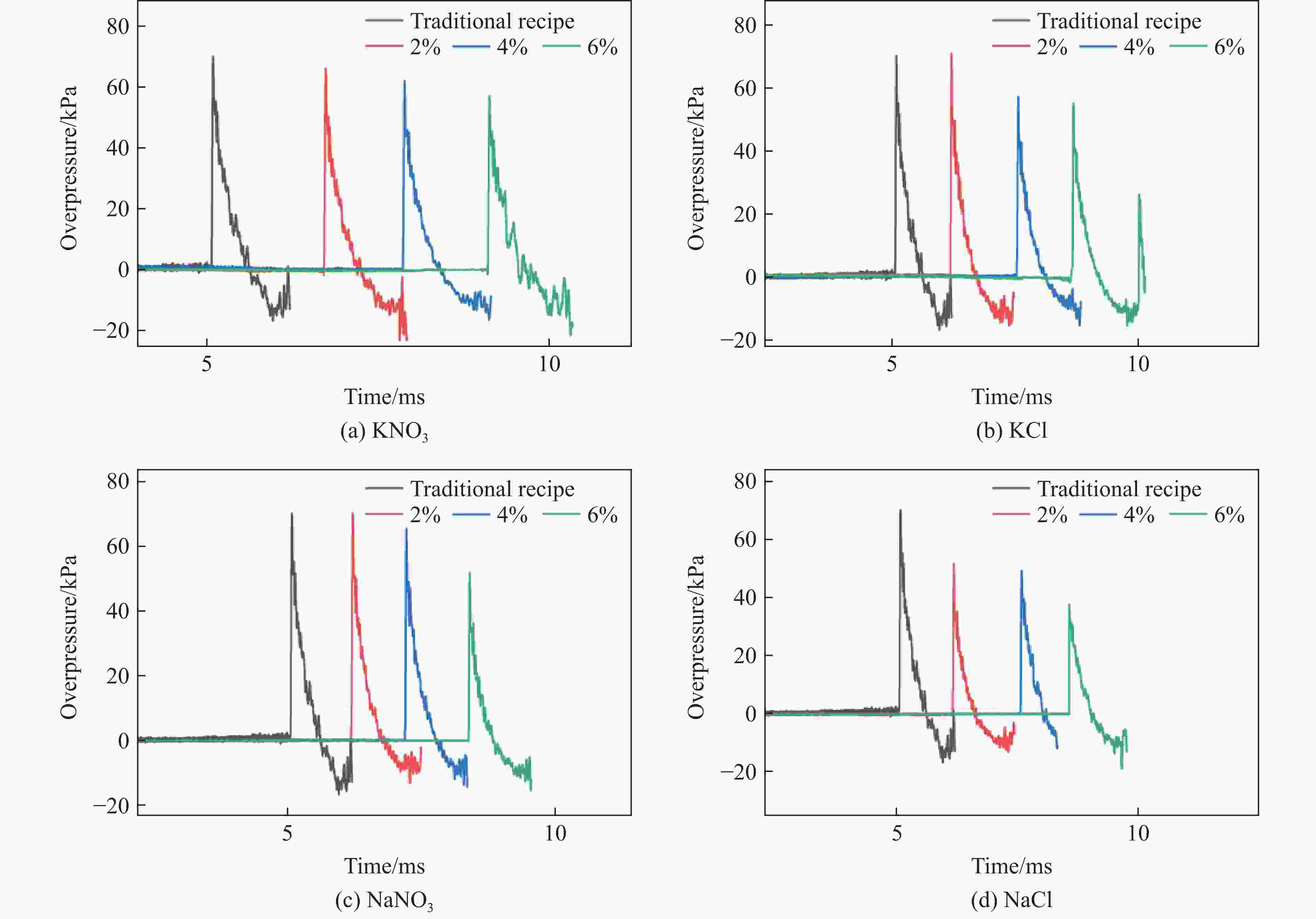
摘要: 为了探究无机盐对硝酸铵溶解温度和膨化硝铵炸药爆炸性能的影响,分别添加不同质量分数(2%、4%和6%)的NaCl、KCl、NaNO3和KNO3 4种无机盐取代膨化硝铵炸药中的硝酸铵,测得了硝酸铵溶解温度和膨化硝铵炸药爆炸性能(爆速、猛度、做功能力)的影响。结果表明:添加无机盐质量分数为2%时,改良炸药较传统膨化硝铵配方炸药的溶解温度降低了8~12 ℃;添加NaNO3和KNO3的膨化硝铵炸药较传统配方炸药的爆速提高了120~150 m/s,添加NaCl和KCl的膨化硝铵炸药降低了150~850 m/s;添加NaNO3和KNO3的膨化硝铵炸药铅柱压缩量较传统配方增大了0.62~1.90 mm,添加NaCl和KCl的铅柱压缩量降低了0.06~2.55 mm;添加NaNO3和KCl的炸药超压峰值提高了0.02~0.78 kPa,添加NaCl和KNO3的炸药超压峰值降低了5.02~19.57 kPa。无机盐的质量分数每提高2%,溶解温度降低7~10℃,爆速降低100~300 m/s,铅柱压缩量缩小0.08~0.73 mm,超压峰值降低1.77~13.50 kPa。实际操作中,可以在膨化硝铵炸药中添加少量NaNO3,这样既有利于降低硝酸铵的溶解温度,同时有利于提高炸药的爆炸性能。Abstract: In order to explore the influence of inorganic salts on the dissolution temperature of ammonium nitrate and the explosive performance of expanded ammonium nitrate explosives, the inorganic salts of 2%, 4% and 6% of NaCl, KCl, NaNO3 and KNO3 respectively were used to replace the ammonium nitrate content in the expanded ammonium nitrate explosives, and the explosion performance (including the detonation velocity, the fierceness, and the work ability were measured). The results show that when the mass fraction of inorganic salt is 2%, the dissolution temperature is 8 to 12 ℃ lower than that of the traditional puffed ammonium nitrate formula explosives; the expanded ammonium nitrate explosives with NaNO3 and KNO3 is 120−150 m/s higher than the traditional formula, and NaCl and KCl are reduced by 150−850 m/s; lead column compression of NaNO3 and KNO3 increased by 0.62−1.90 mm, and NaCl and KCl decreased by 0.06−2.55 mm; the peak overpressure of NaNO3 and KCl increased by 0.02−0.78 kPa, and NaCl and KNO3 inorganic salts decreased by 5.02−19.57 kPa. For every 2% increase in the mass fraction of inorganic salt substitution, the dissolution temperature decreases by 7 to 10 ℃; the detonation velocity decreases by 100 to 300 m/s; lead column compression decreases by 0.08 to 0.73 mm; and the peak overpressure decrease by 1.77 to 13.5 kPa. In practice, a small quantity of NaNO3 can be added to the expanded ammonium nitrate explosives, which is not only conducive to reducing the dissolution temperature of ammonium nitrate, but also enhances the explosive performance of explosives.
-
表 1 不同质量分数、不同无机盐改性膨化硝铵炸药的氧平衡
Table 1. Oxygen balance of expanded ammonium nitrate explosive modified by inorganic salt composition with different mass fractions
Composition OB/(g·g−1) ω=2% ω=4% ω=6% NaNO3 − 0.0030 0.0024 − 0.0030 KNO3 − 0.0045 − 0.0006 − 0.0045 KCl − 0.0124 − 0.0164 − 0.0124 NaCl − 0.0124 − 0.0164 − 0.0124 表 2 不同质量分数、不同无机盐改性膨化硝铵炸药的爆热
Table 2. Detonation heat of inorganic salt-modified expanded ammonium nitrate explosives with different mass fractions and components
Composition Detonation heat/(kJ·mol−1) ω=2% ω=4% ω=6% NaNO3 3696.86 3564.76 3375.36 KNO3 3728.61 3562.03 3371.26 KCl 3688.90 3586.73 3484.56 NaCl 3688.90 3586.73 3484.56 表 3 不同质量分数、不同无机盐改性膨化硝铵炸药完全溶解时的温度
Table 3. Temperature when inorganic salt modified expanded ammonium nitrate explosives with different mass fractions and components are completely dissolved
Composition Temperature/℃ ω=2% ω=4% ω=6% NaNO3 112 105 95 KNO3 110 100 90 KCl 110 102 92 NaCl 108 101 95 表 4 不同配比改性膨化硝铵炸药的爆速
Table 4. Detonation velocity for each ratio of explosives
Composition Detonation velocity/(m·s−1) Error of detonation velocity/(m·s−1) ω=2% ω=4% ω=6% ω=2% ω=4% ω=6% NaNO3 2983.29 2666.67 2364.18 45.36 56.38 49.65 KNO3 2981.52 2665.44 2393.90 60.49 62.68 52.89 KCl 2708.51 2468.60 2156.15 43.59 75.81 38.54 NaCl 1999.20 1786.99 1667.33 61.56 52.56 54.87 表 5 各配比膨化硝铵炸药的超压峰值
Table 5. Overpressure peak of each ratio of expanded ammonium nitrate explosives
Composition Overpressure peak/kPa Error of overpressure peak/kPa ω=2% ω=4% ω=6% ω=2% ω=4% ω=6% NaNO3 70.19 65.44 51.91 0.76 0.85 0.72 KNO3 66.15 62.13 57.17 1.02 0.68 0.81 KCl 70.95 57.22 55.11 0.83 1.16 0.89 NaCl 51.70 49.43 37.67 0.68 0.77 0.68 表 6 各配比膨化硝铵炸药的最大冲量
Table 6. Maximum impulse of each ratio of expanded ammonium nitrate explosives
Composition Maximum impulse/(Pa·s) Error of maximum impulse/(Pa·s) ω=2% ω=4% ω=6% ω=2% ω=4% ω=6% NaNO3 13.08 12.19 11.35 0.33 0.13 0.12 KNO3 12.95 11.29 10.86 0.08 0.29 0.09 KCl 11.88 11.26 10.86 0.19 0.16 0.14 NaCl 10.70 9.86 8.85 0.09 0.09 0.18 -
[1] HE Z W, LI Y Y, YU Y K, et al. Preparation and performance of ANPyO/emulsion explosive composite energetic system [J]. Propellants, Explosives, Pyrotechnics, 2022, 47(11): e202200121. doi: 10.1002/prep.202200121 [2] ZHANG K M, ZHAO H R. Perspectives in the stability of emulsion explosive [J]. Advances in Colloid and Interface Science, 2022, 307: 102745. doi: 10.1016/j.cis.2022.102745 [3] CHOU J P, MA H H, WANG Y X, et al. Effect of hydrogen-storage pressure on the detonation characteristics of emulsion explosives sensitized by glass microballoons [J]. Defence Technology, 2022, 18(5): 747–754. doi: 10.1016/j.dt.2021.03.021 [4] 孙彦臣, 黄文尧, 梁昊, 等. 硝酸铵细度对铵胺炸药性能的影响 [J]. 火炸药学报, 2024, 47(4): 316–323. doi: 10.14077/j.issn.1007-7812.202308017SUN Y C, HUANG W Y, LIANG H, et al. Effect of fineness of ammonium nitrate on properties of ammonium amine explosive [J]. Chinese Journal of Explosives & Propellants, 2024, 47(4): 316–323. doi: 10.14077/j.issn.1007-7812.202308017 [5] 孙占辉, 孙金华, 邵荃. 盐酸对硝酸铵热分解的影响 [J]. 中国科学技术大学学报, 2006, 36(4): 370–373. doi: 10.3969/j.issn.0253-2778.2006.04.005SUN Z H, SUN J H, SHAO Q. On the decomposition of AN catalyzed by HCl [J]. Journal of University of Science and Technology of China, 2006, 36(4): 370–373. doi: 10.3969/j.issn.0253-2778.2006.04.005 [6] 王志荣, 胡园园, 吴倩. 敞开气氛中硝酸铵热分解过程危险特性的实验研究 [J]. 中国安全生产科学技术, 2010, 6(1): 49–53. doi: 10.3969/j.issn.1673-193X.2010.01.011WANG Z R, HU Y Y, WU Q. Experimental study on hazard characteristics during thermal decomposition process of ammonium nitrate in opened atmosphere [J]. Journal of Safety Science and Technology, 2010, 6(1): 49–53. doi: 10.3969/j.issn.1673-193X.2010.01.011 [7] 白燕. 硝酸铵水溶液热稳定性研究 [J]. 工业安全与环保, 2009, 35(8): 4–6. doi: 10.3969/j.issn.1001-425X.2009.08.002BAI Y. Study of the thermal stability of ammonium nitrate in aqueous solution [J]. Industrial Safety and Environmental Protection, 2009, 35(8): 4–6. doi: 10.3969/j.issn.1001-425X.2009.08.002 [8] 曹会琦. 不同种类添加剂对硝酸铵热分解动力学及热危险性的影响研究 [D]. 合肥: 中国科学技术大学, 2022.CAO H Q. Research on the effect of different kinds of additives on the thermal decomposition kinetics and thermal risk of ammonium nitrate [D]. Hefei: University of Science and Technology of China, 2022. [9] 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 工业炸药爆速测定方法:GB/T 13228—2015 [S]. 北京: 中国标准出版社, 2015.General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China. Test method of detonation velocity for industrial explosive: GB/T 13228—2015 [S]. Beijing: China Standard Press, 2015. [10] 国家技术监督局. 炸药猛度试验 铅柱压缩法:GB/T 12440—1990 [S]. 北京: 中国标准出版社, 1990.The State Bureau of Quality and Technical Supervision. Explosive-determination of brisance-lead cylinder compression test: GB/T 12440—1990 [S]. Beijing: China Standard Press, 1990. [11] 张立, 吴红波. 爆破器材测试技术 [M]. 合肥: 中国科学技术大学出版社, 2018.ZHANG L, WU H B. Explosive materials testing technology [M]. Hefei: University of Science and Technology of China Press, 2018. [12] 吕春绪, 刘祖亮, 陆明, 等. 工业炸药理论 [M]. 北京: 兵器工业出版社, 2003.LYU C X, LIU Z L, LU M, et al. Industrial explosive theory [M]. Beijing: The Publishing House of Ordnance Industry, 2003. [13] 陆明. 工业炸药配方设计 [M]. 北京: 北京理工大学出版社, 2002.LU M. Industrial explosive formula design [M]. Beijing: Beijing Institute of Technology Press, 2022. [14] 张为鹏, 张亦安, 赵省向. 杂质的影响及硝铵生产中爆炸事故的预防 [J]. 化肥工业, 2000, 27(1): 40–42, 63. doi: 10.3969/j.issn.1006-7779.2000.01.011ZHANG W P, ZHANG Y A, ZHAO S X. Effect of impurities and prevention of explosion accident in production of ammonium nitrate [J]. Chemical Fertilizer Industry, 2000, 27(1): 40–42, 63. doi: 10.3969/j.issn.1006-7779.2000.01.011 [15] 赵东风, 彭力, 王文东. 无机添加剂对硝酸铵拒爆性的影响研究 [J]. 燃料化学学报, 2006, 34(1): 113–116. doi: 10.3969/j.issn.0253-2409.2006.01.024ZHAO D F, PENG L, WANG W D. Effects of inorganic additives on the misfire of modified ammonium nitrate [J]. Journal of Fuel Chemistry and Technology, 2006, 34(1): 113–116. doi: 10.3969/j.issn.0253-2409.2006.01.024 [16] 夏良洪. 典型添加剂对硝酸铵基础性能的影响研究 [D]. 南京: 南京理工大学, 2015.XIA L H. The effect of typical additives on the basis performance of ammonium nitrate [D]. Nanjing: Nanjing University of Science & Technology, 2015. -






 下载:
下载: