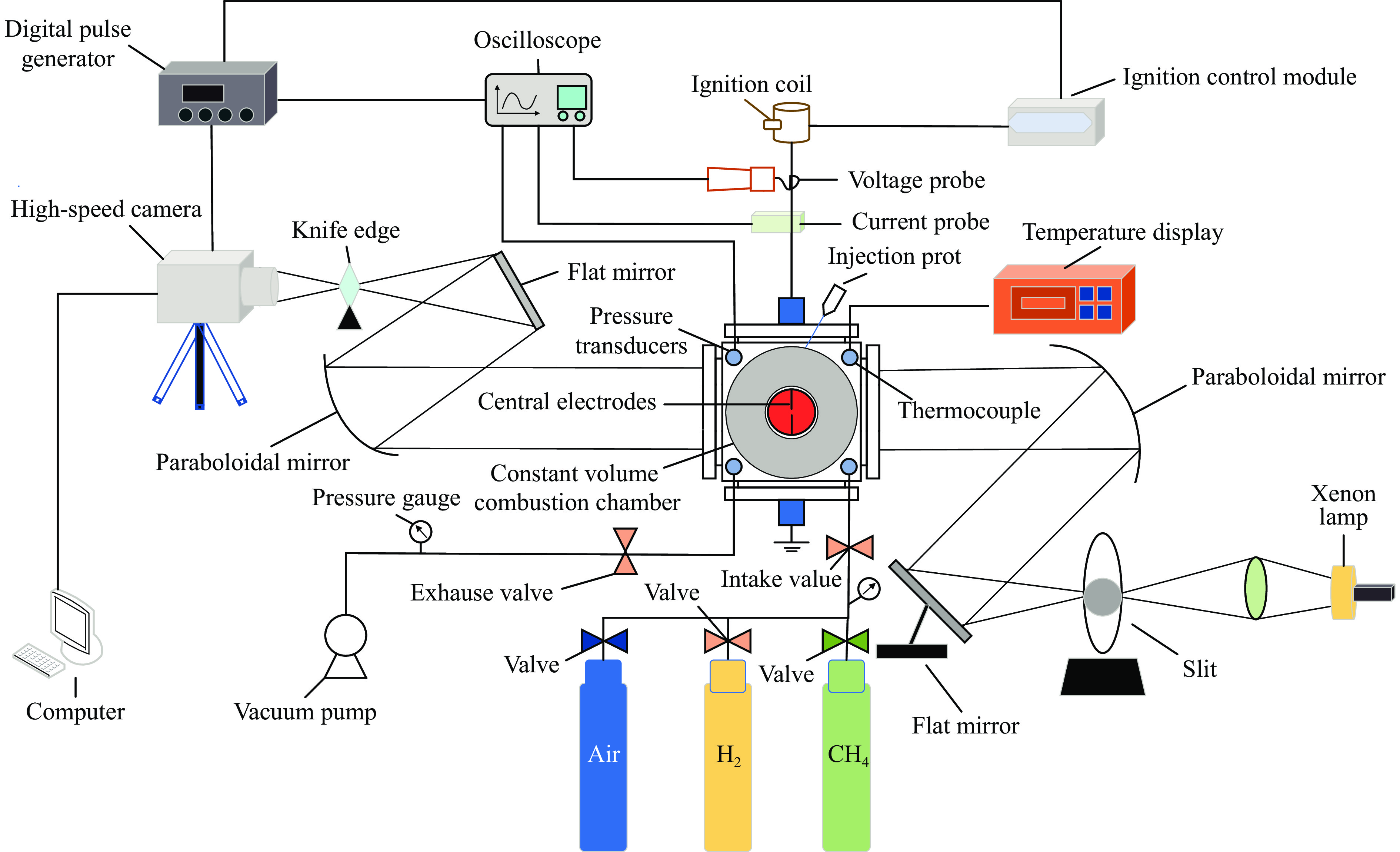
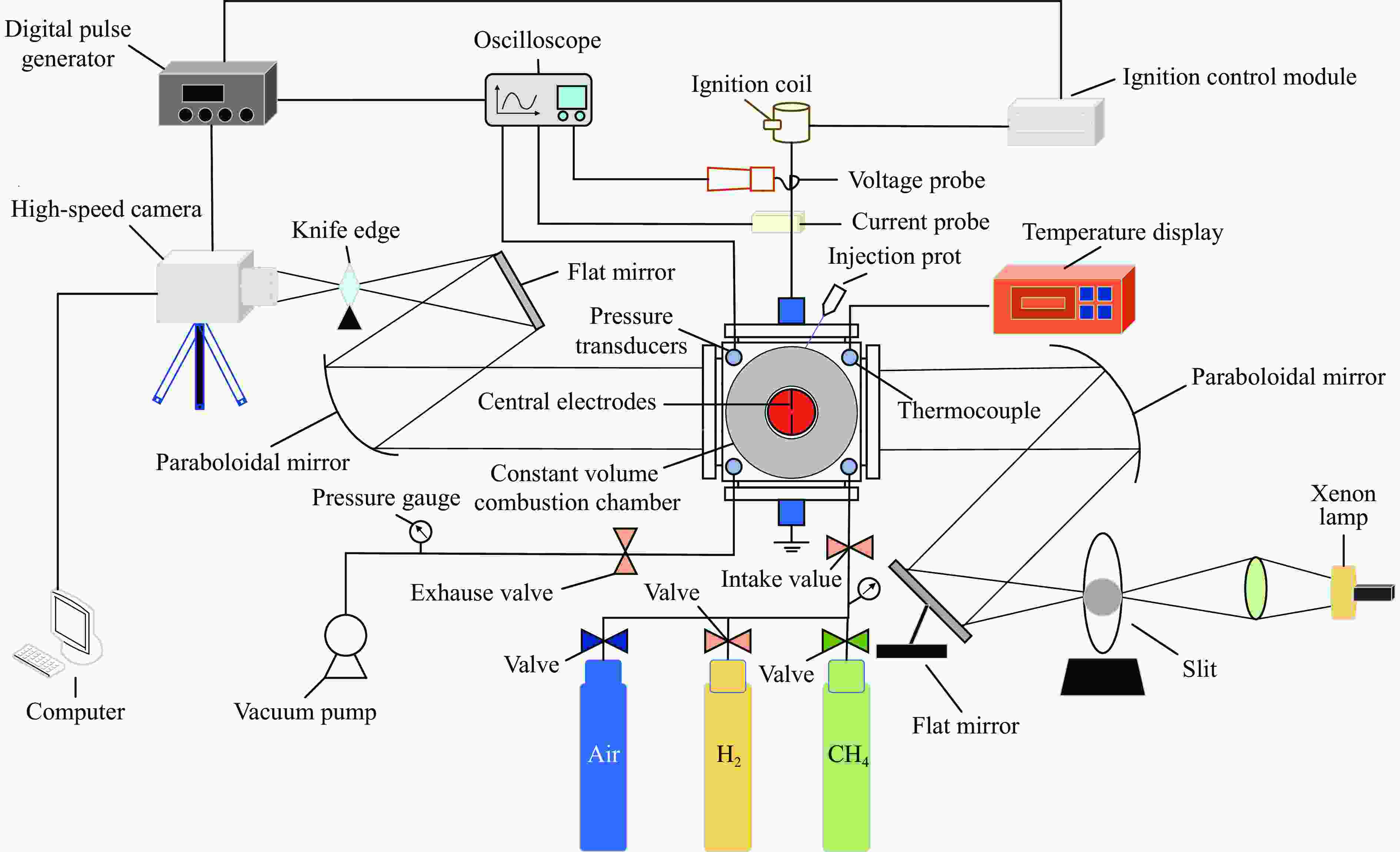
Effect of High Temperature and High Pressure on the Explosion Characteristics of Ternary Premixed Fuel
-
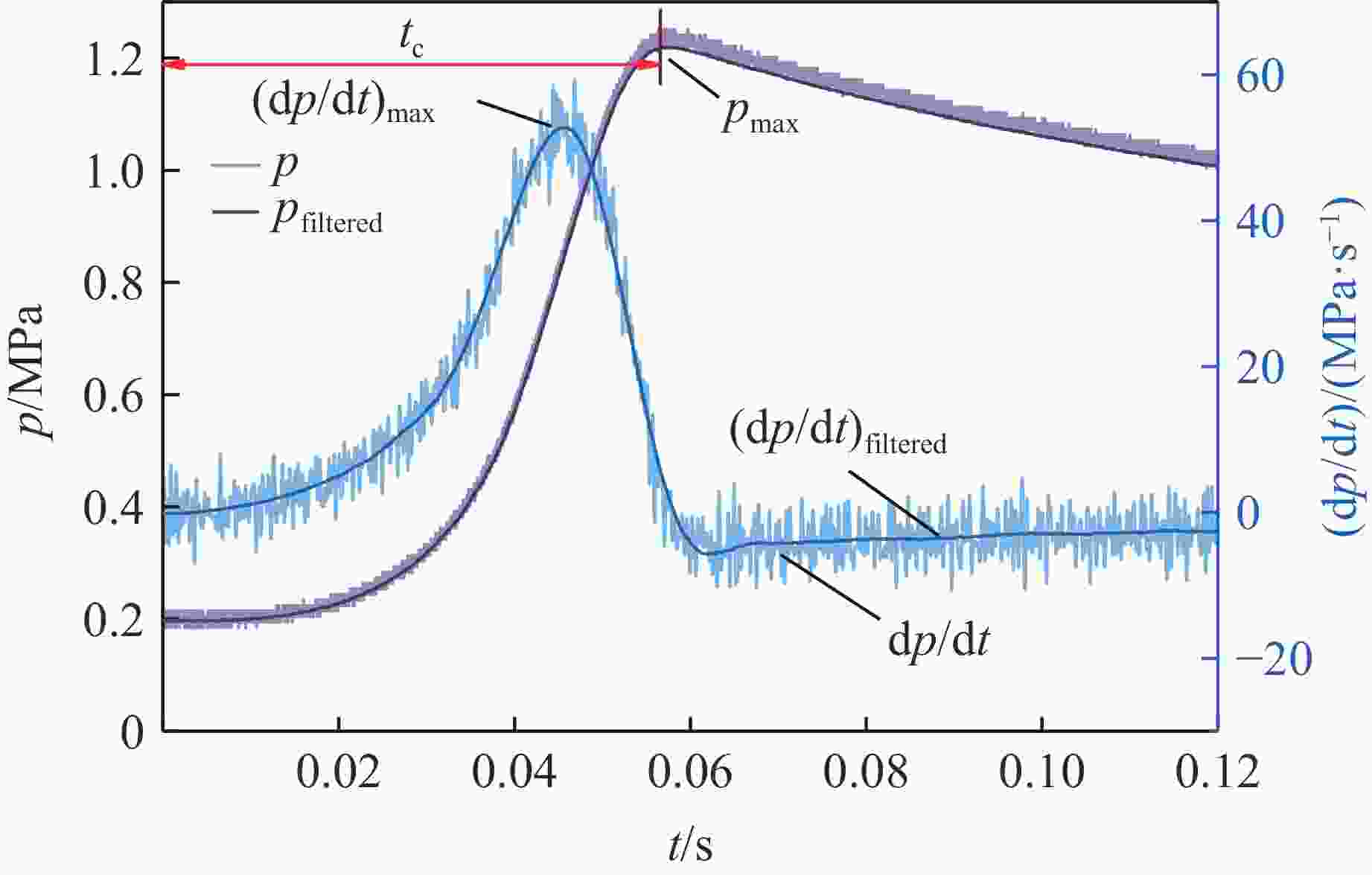
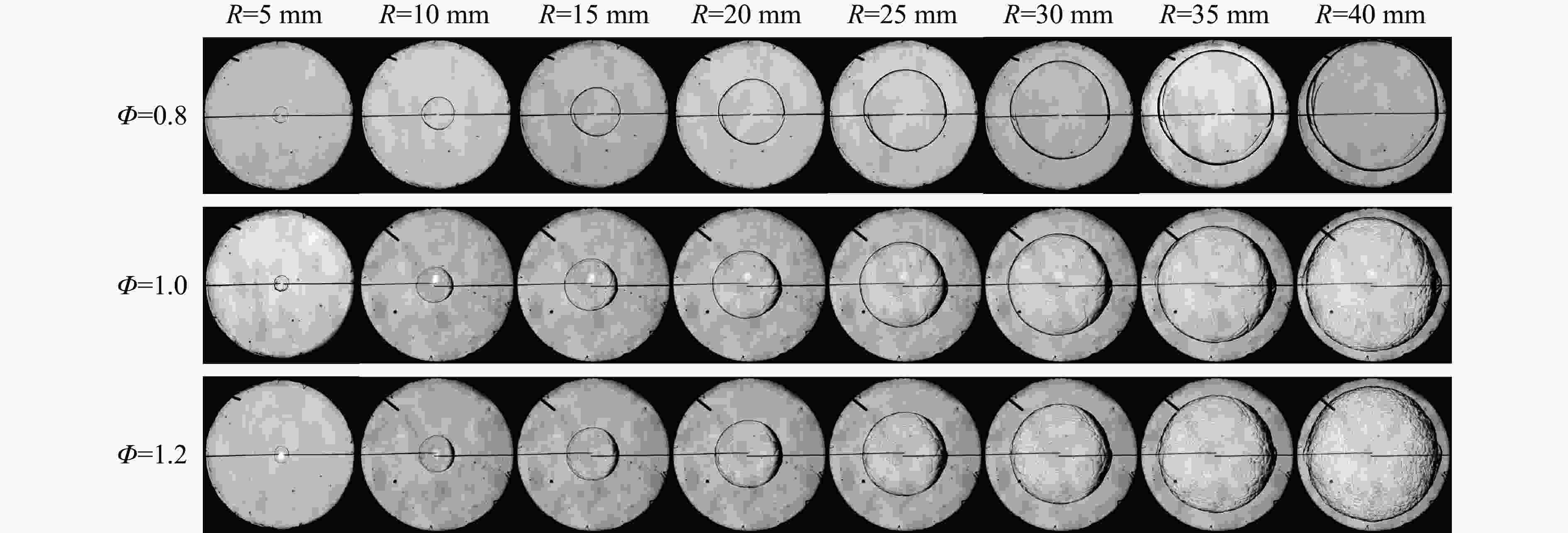
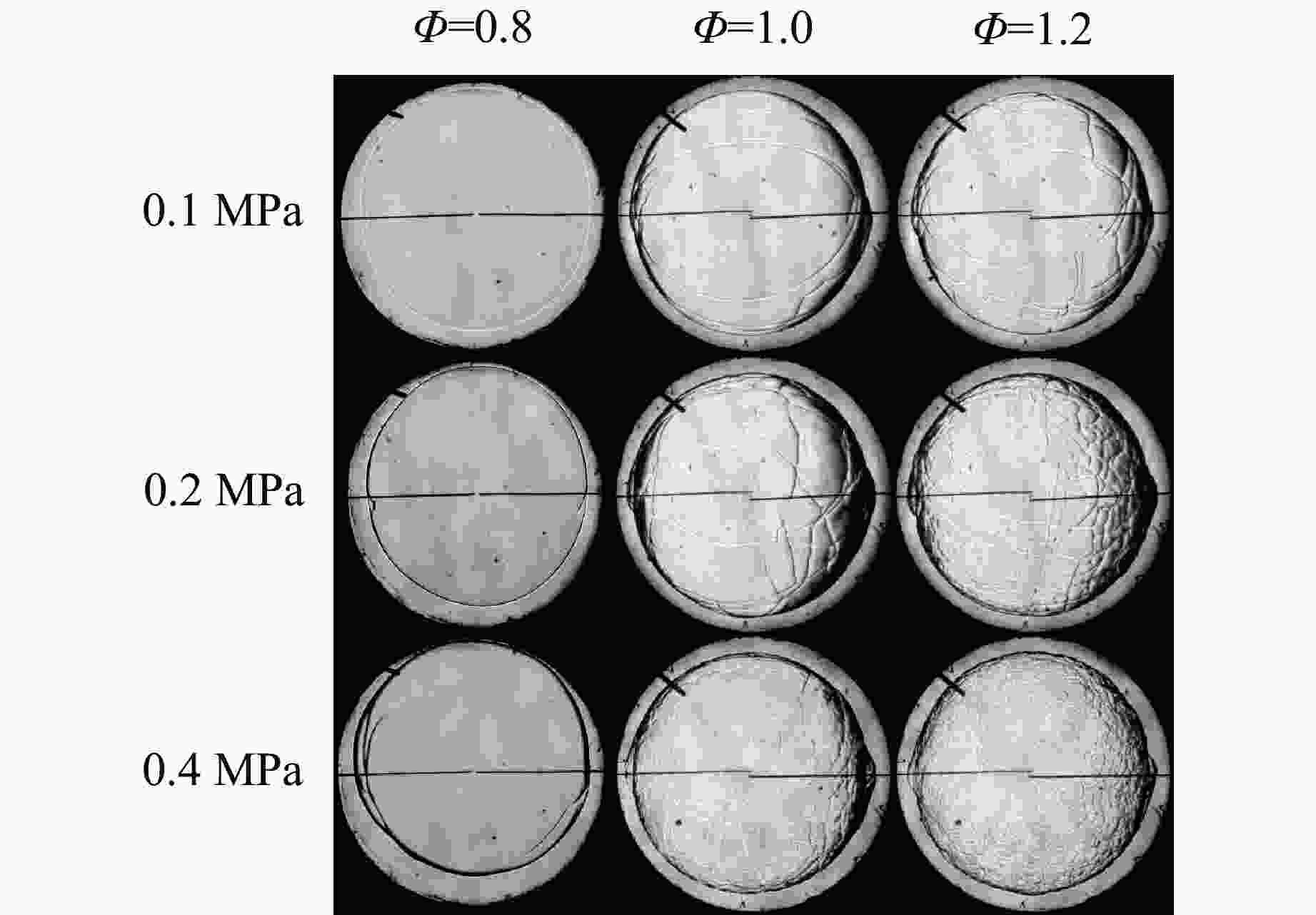
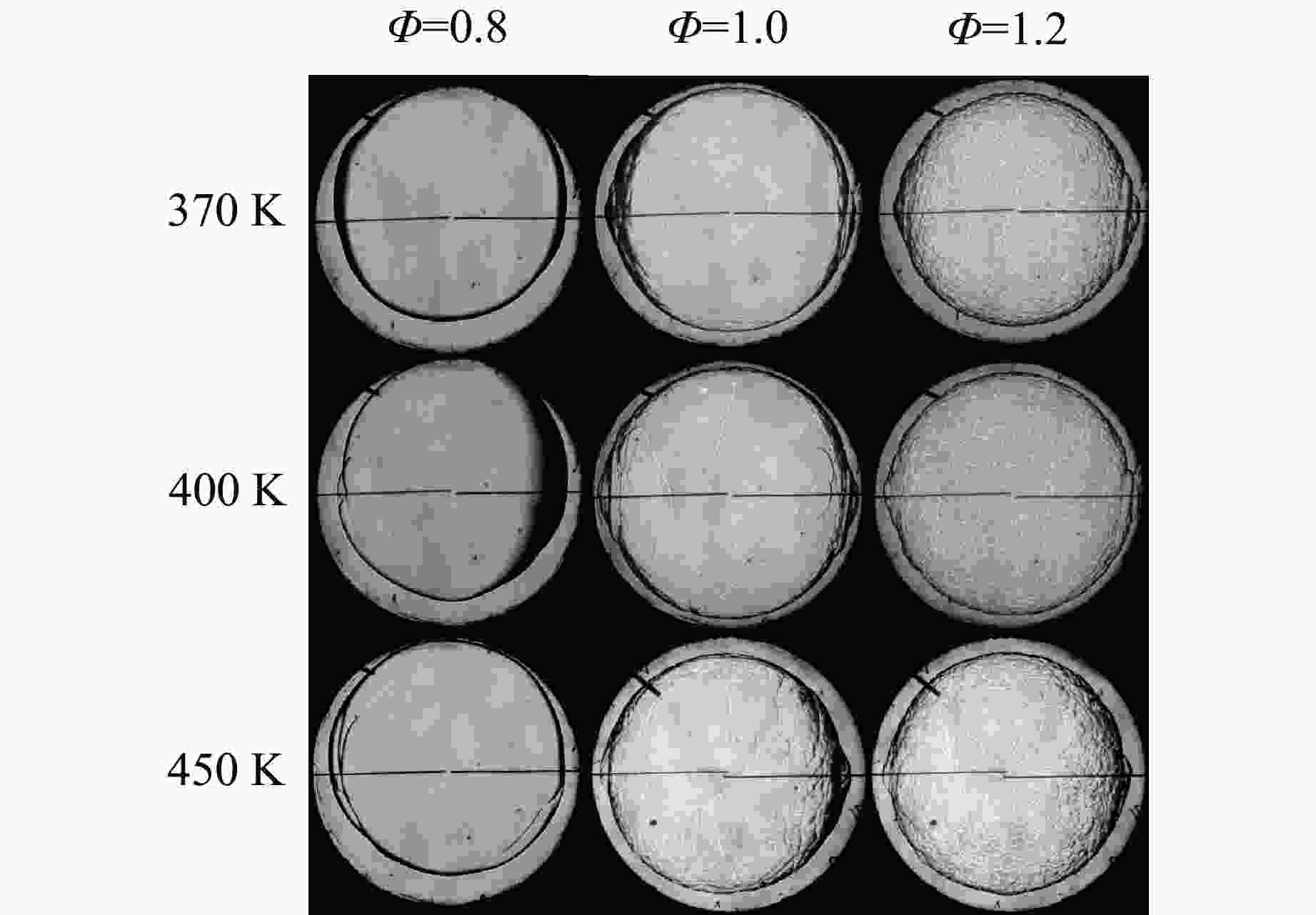
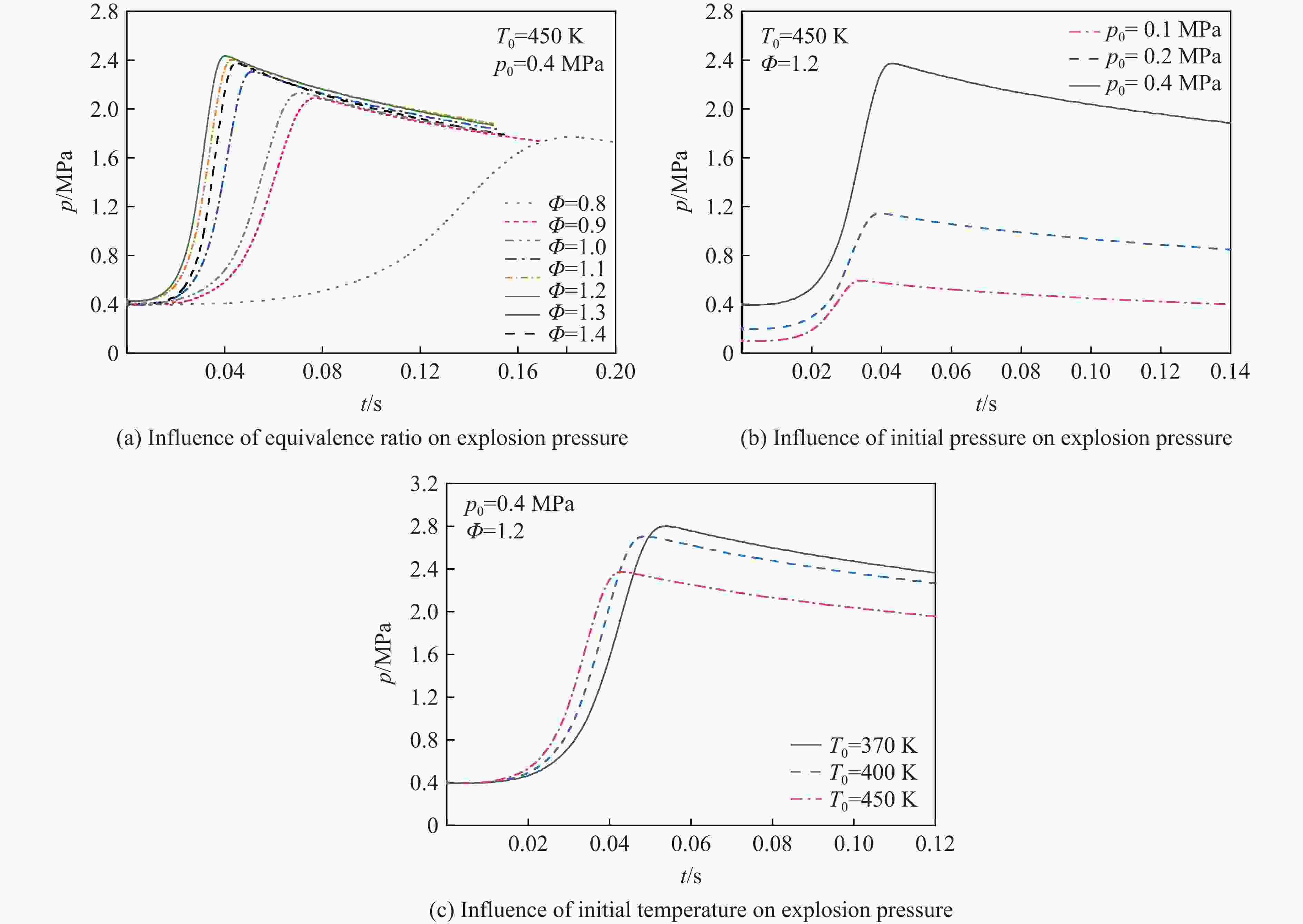
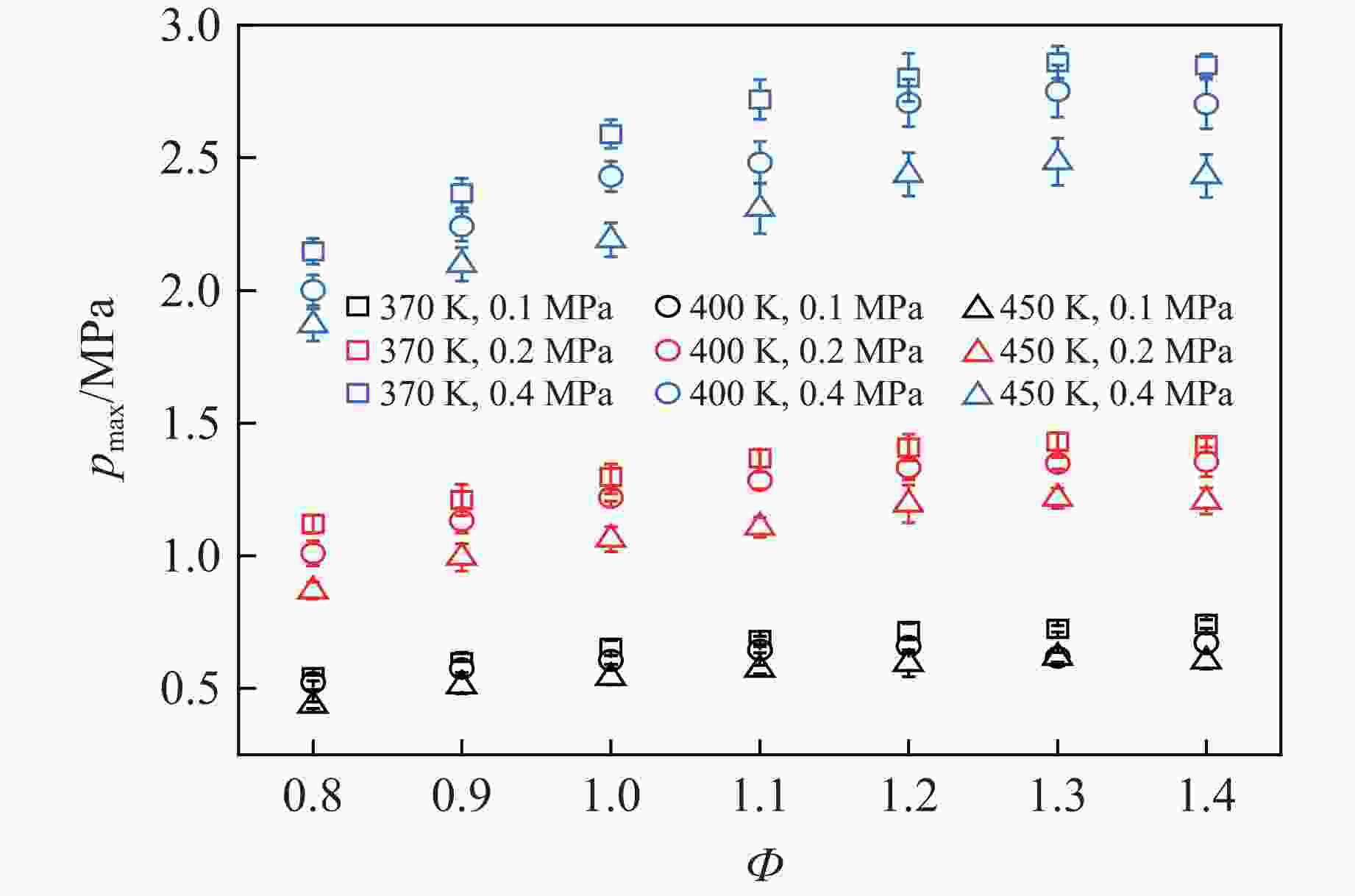
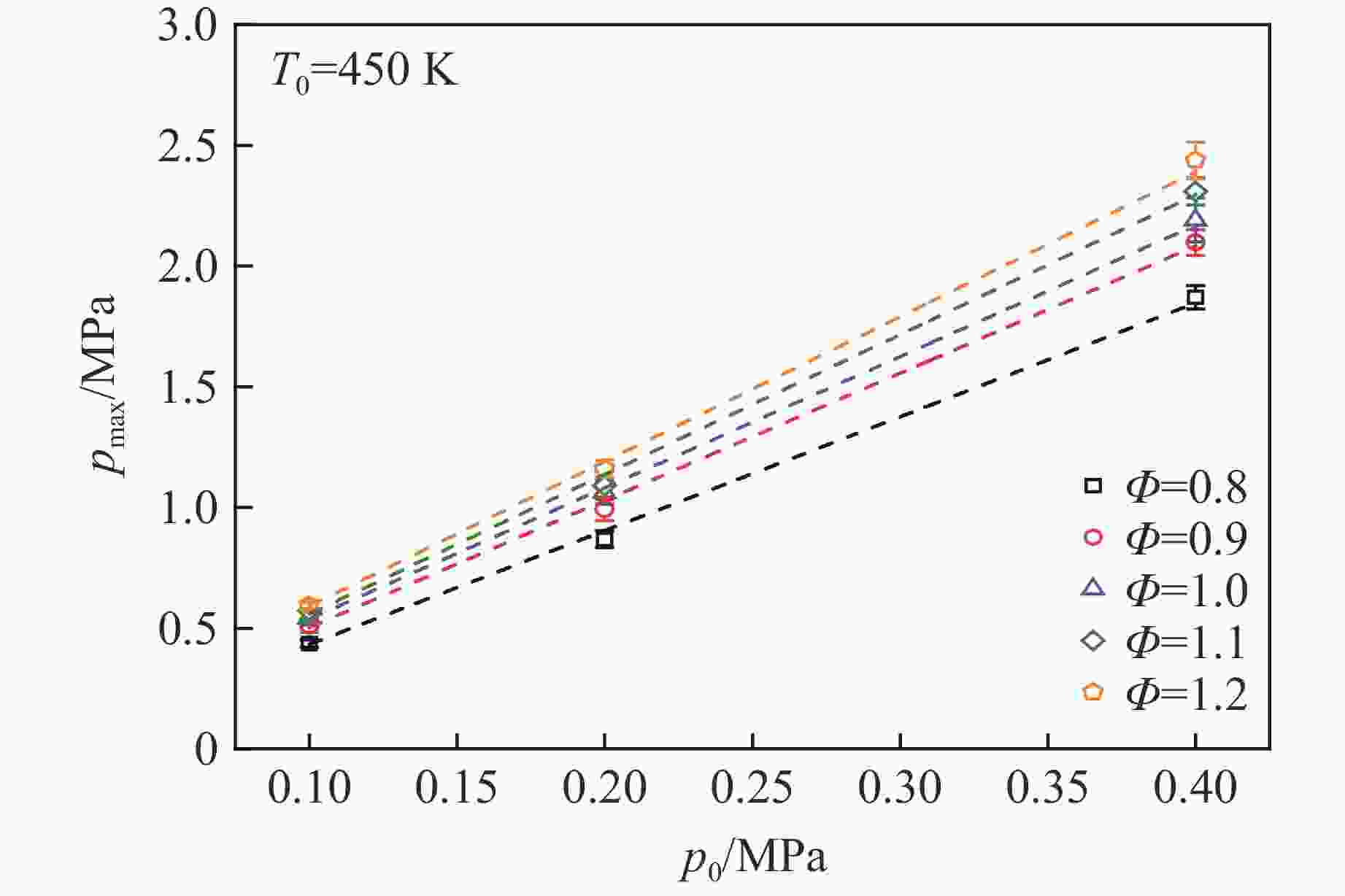
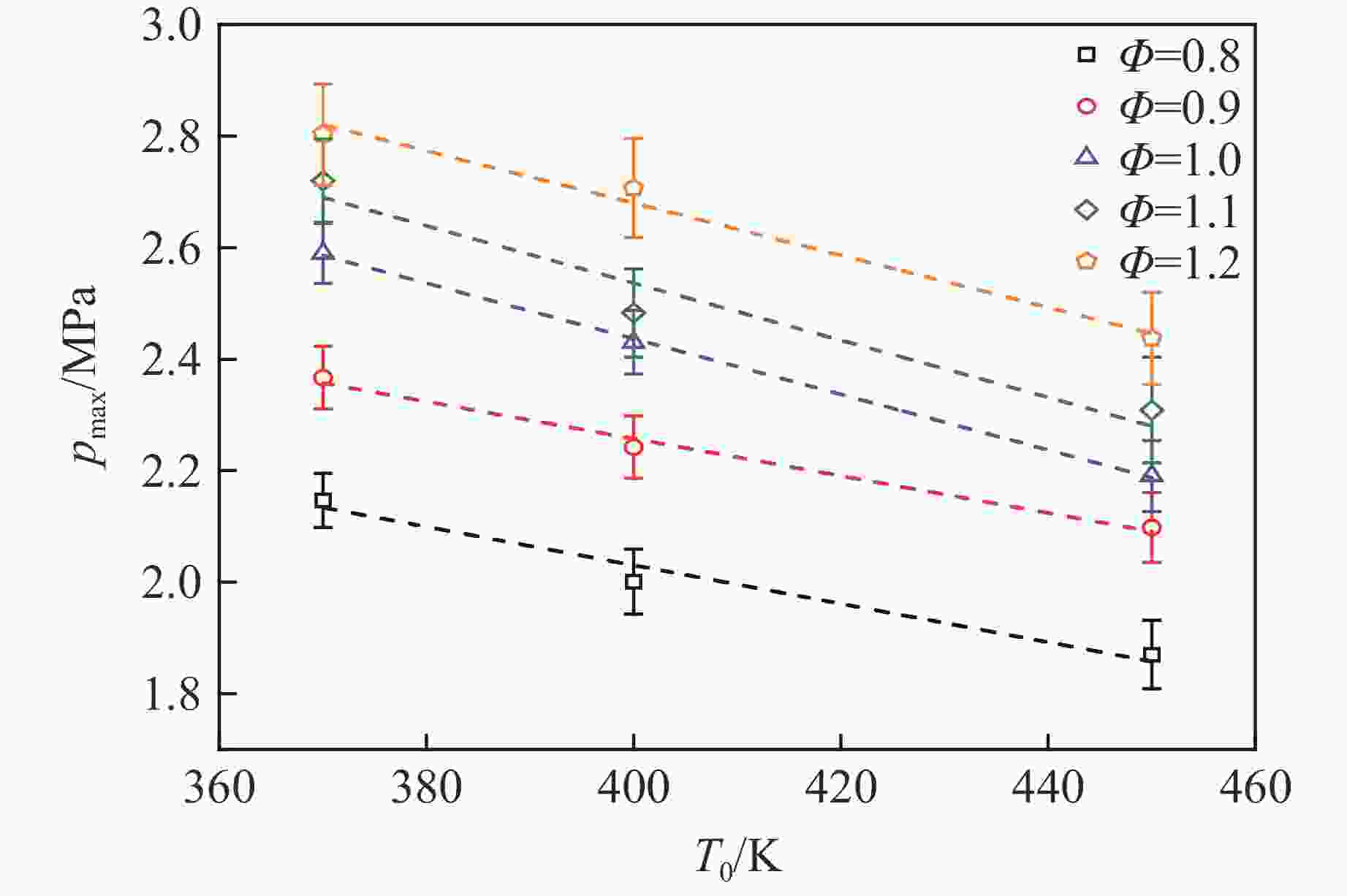
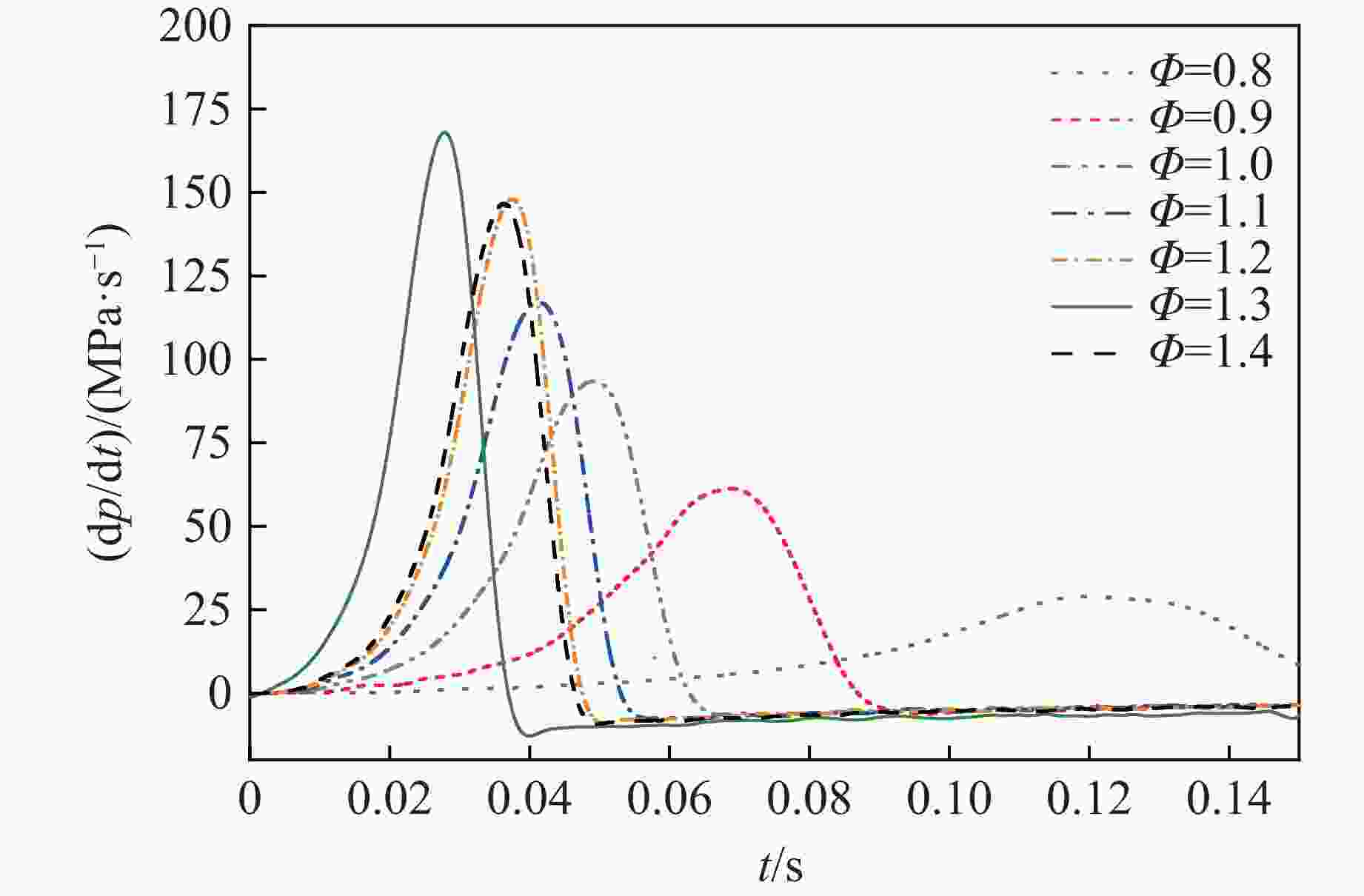
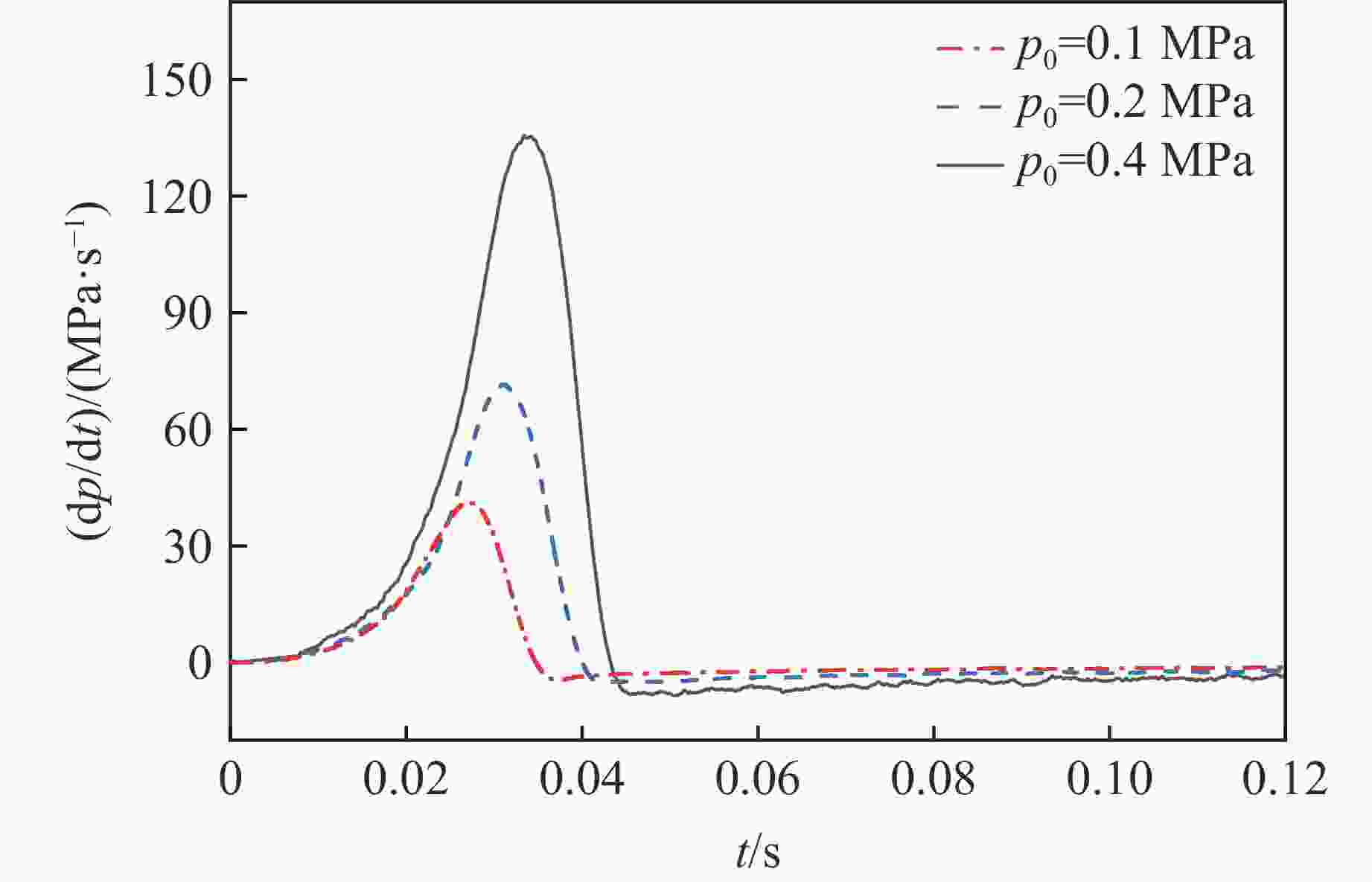
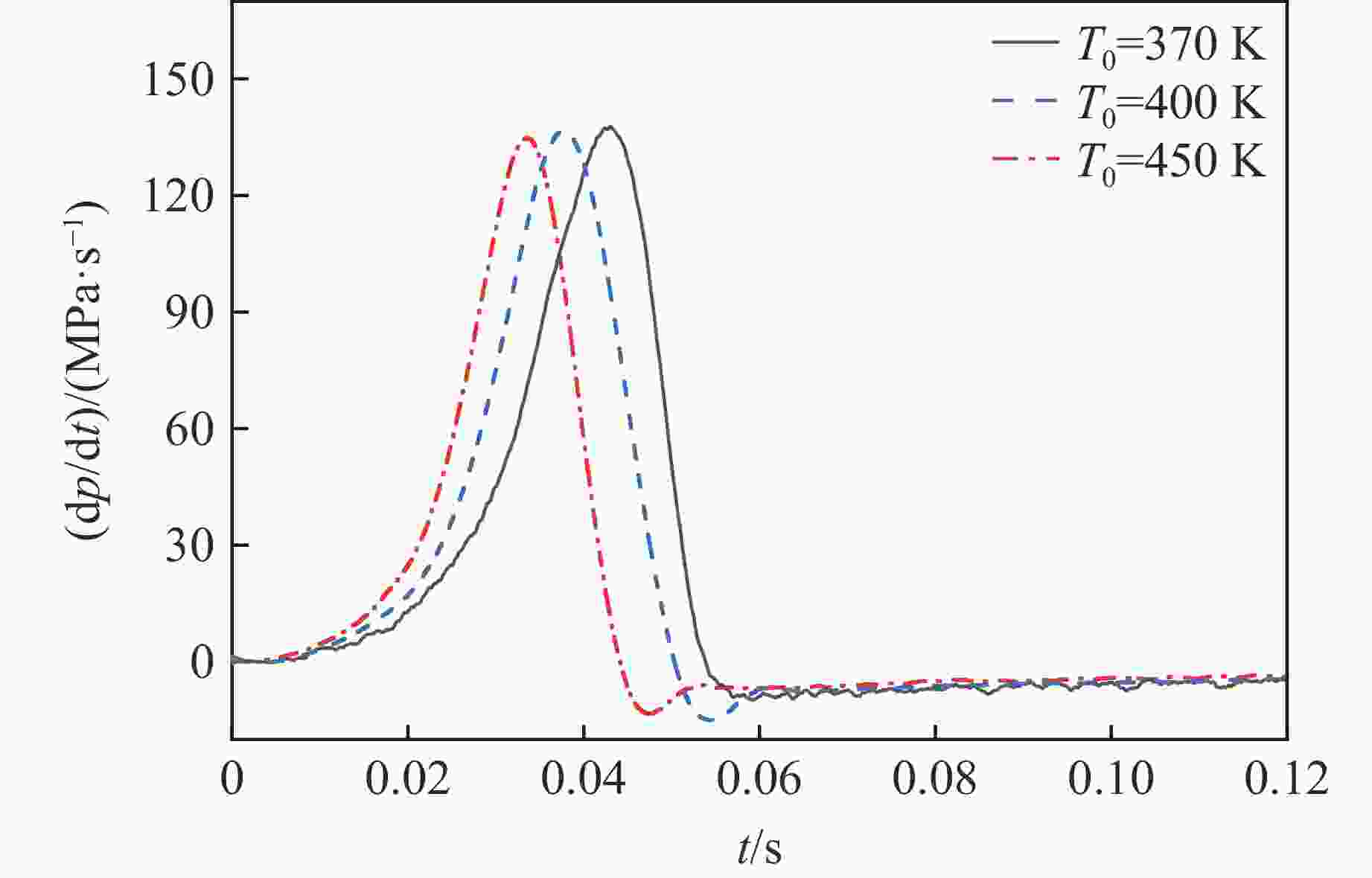
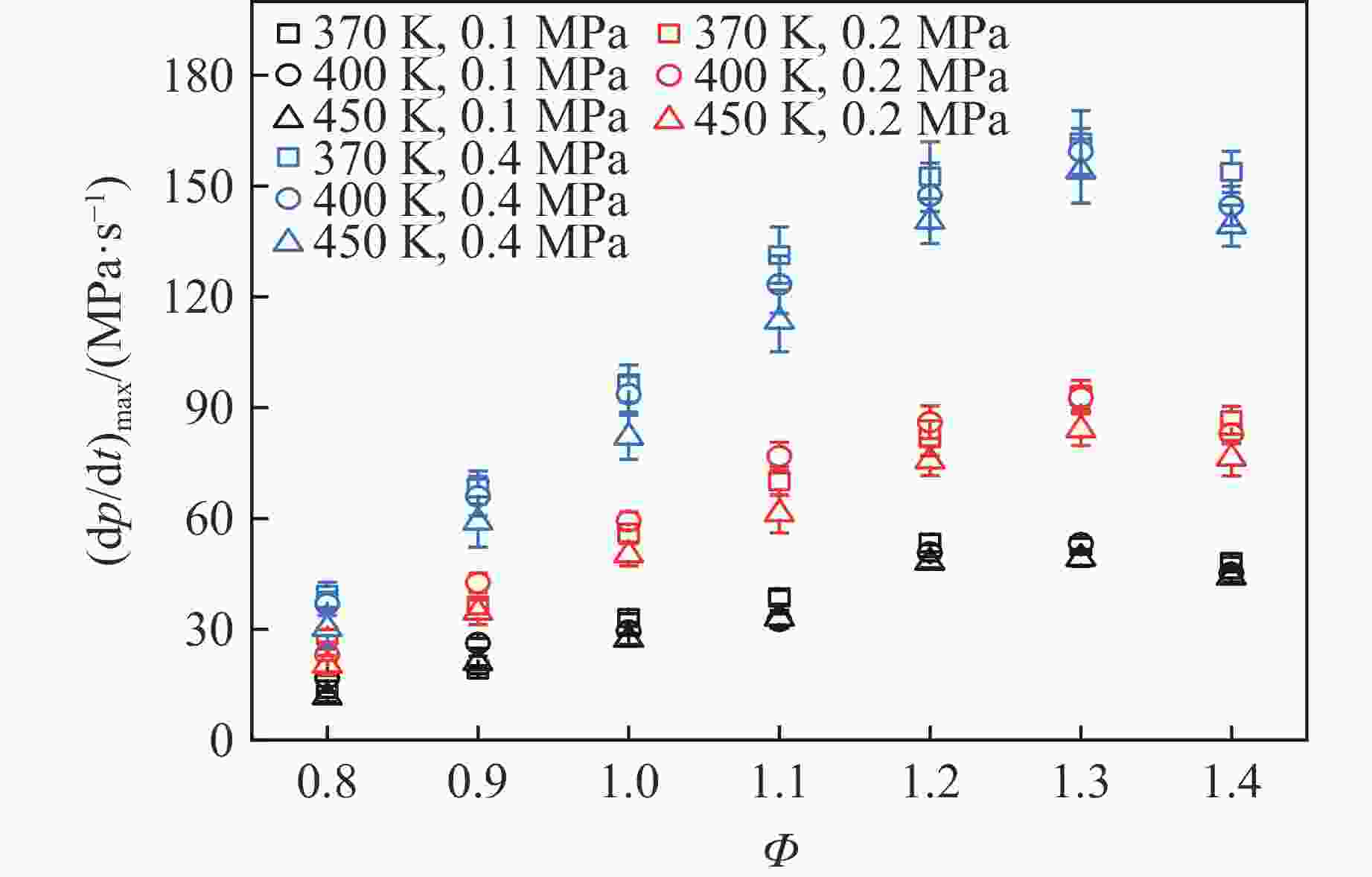
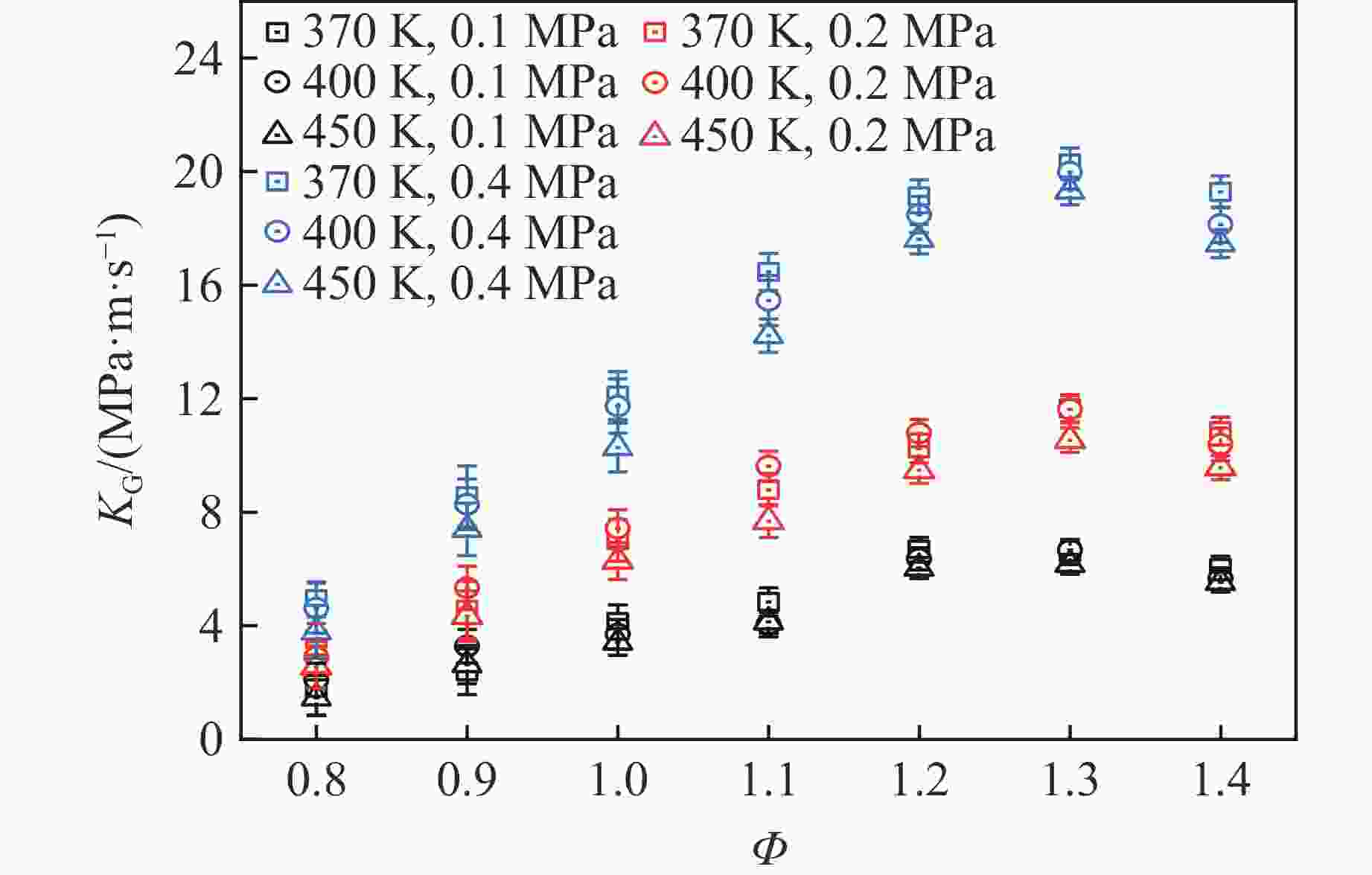
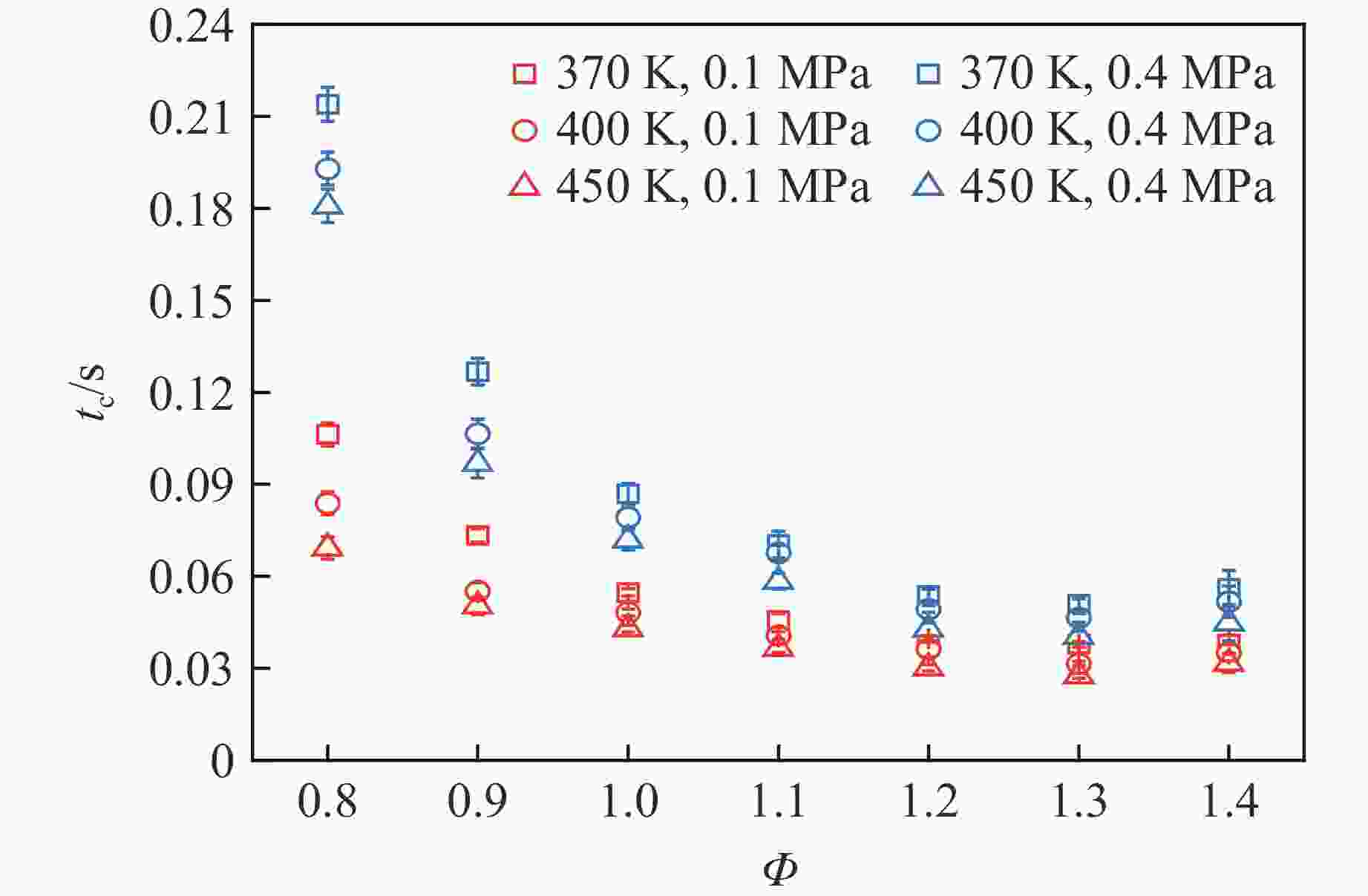
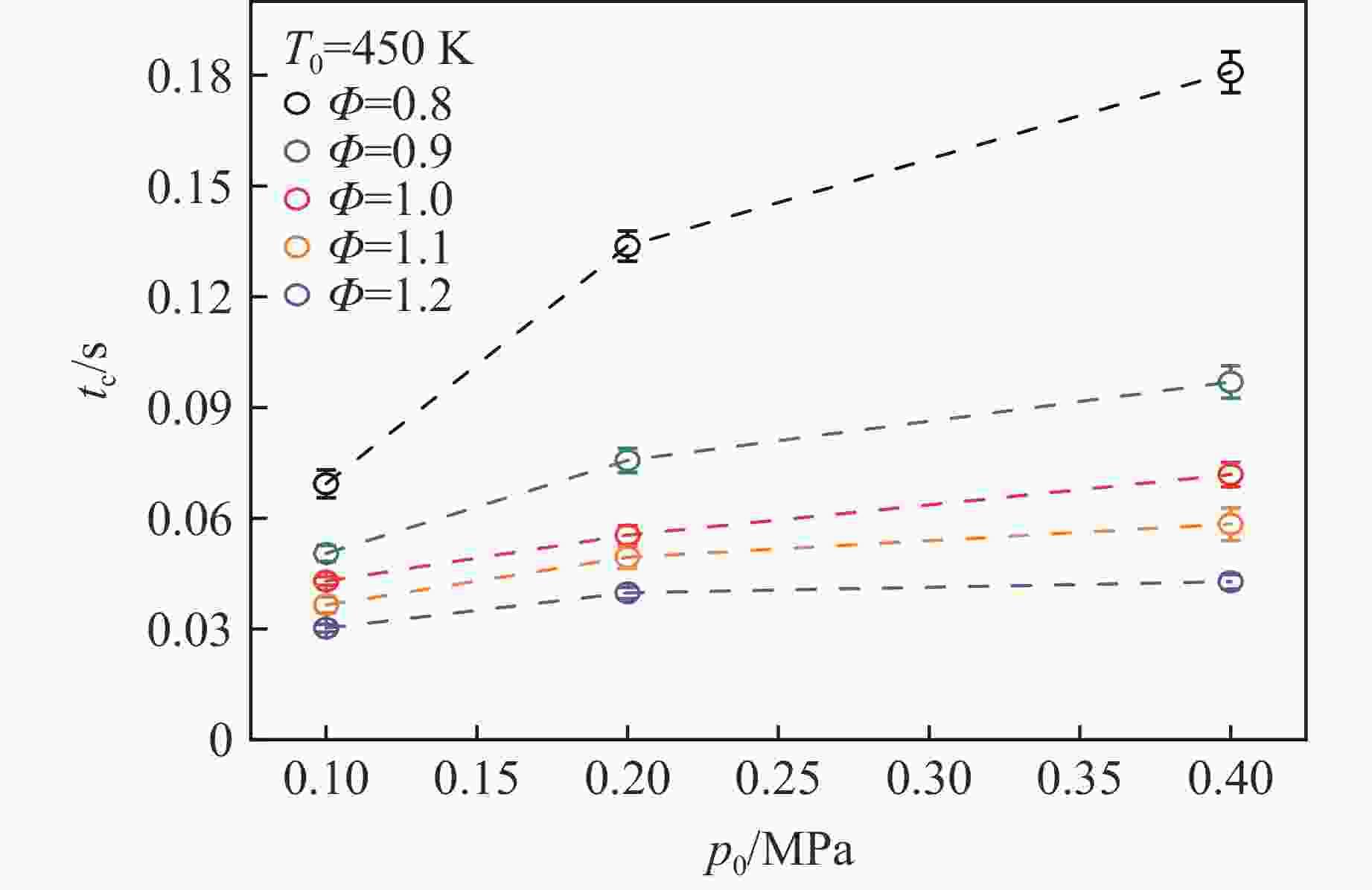
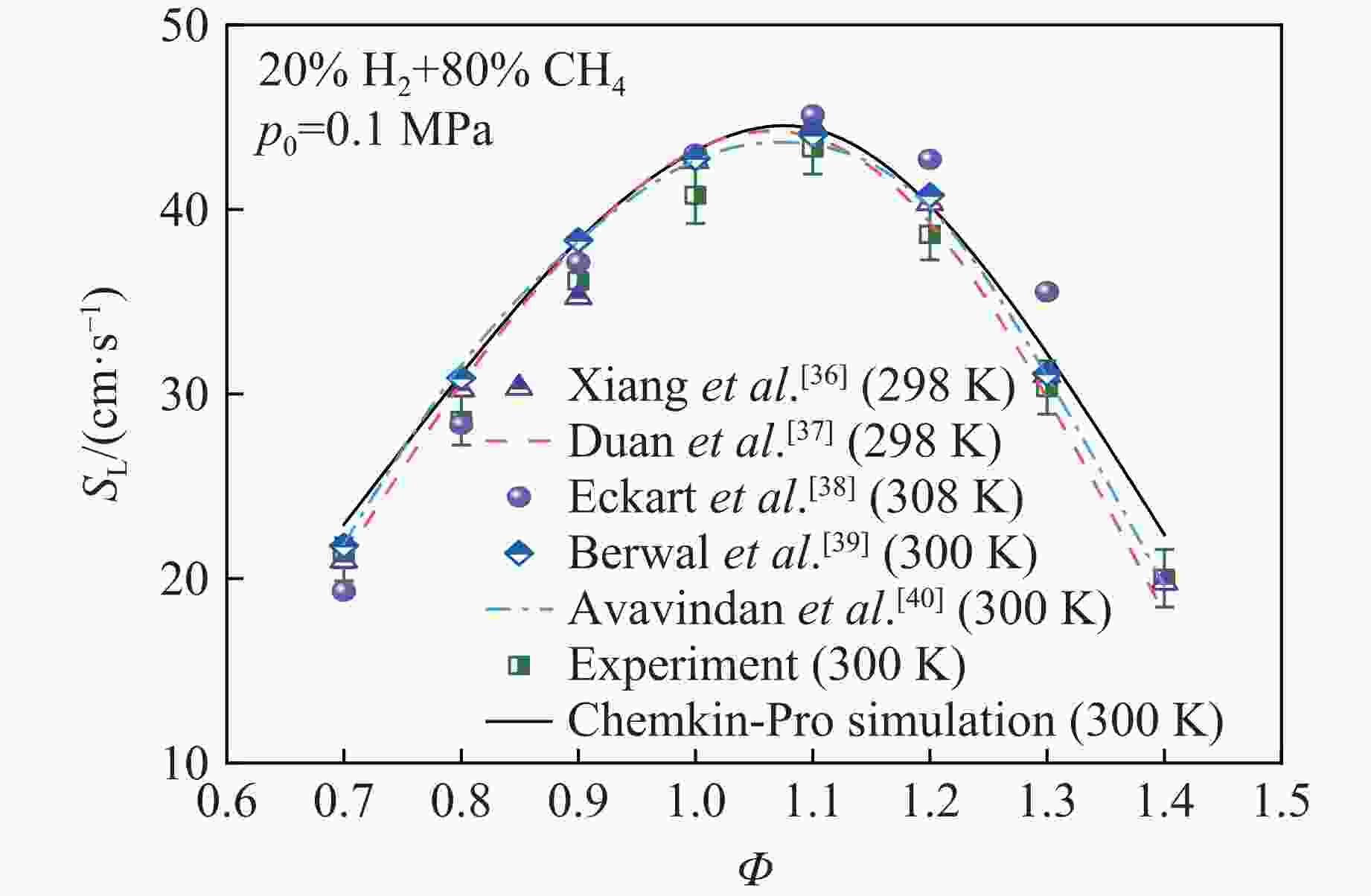
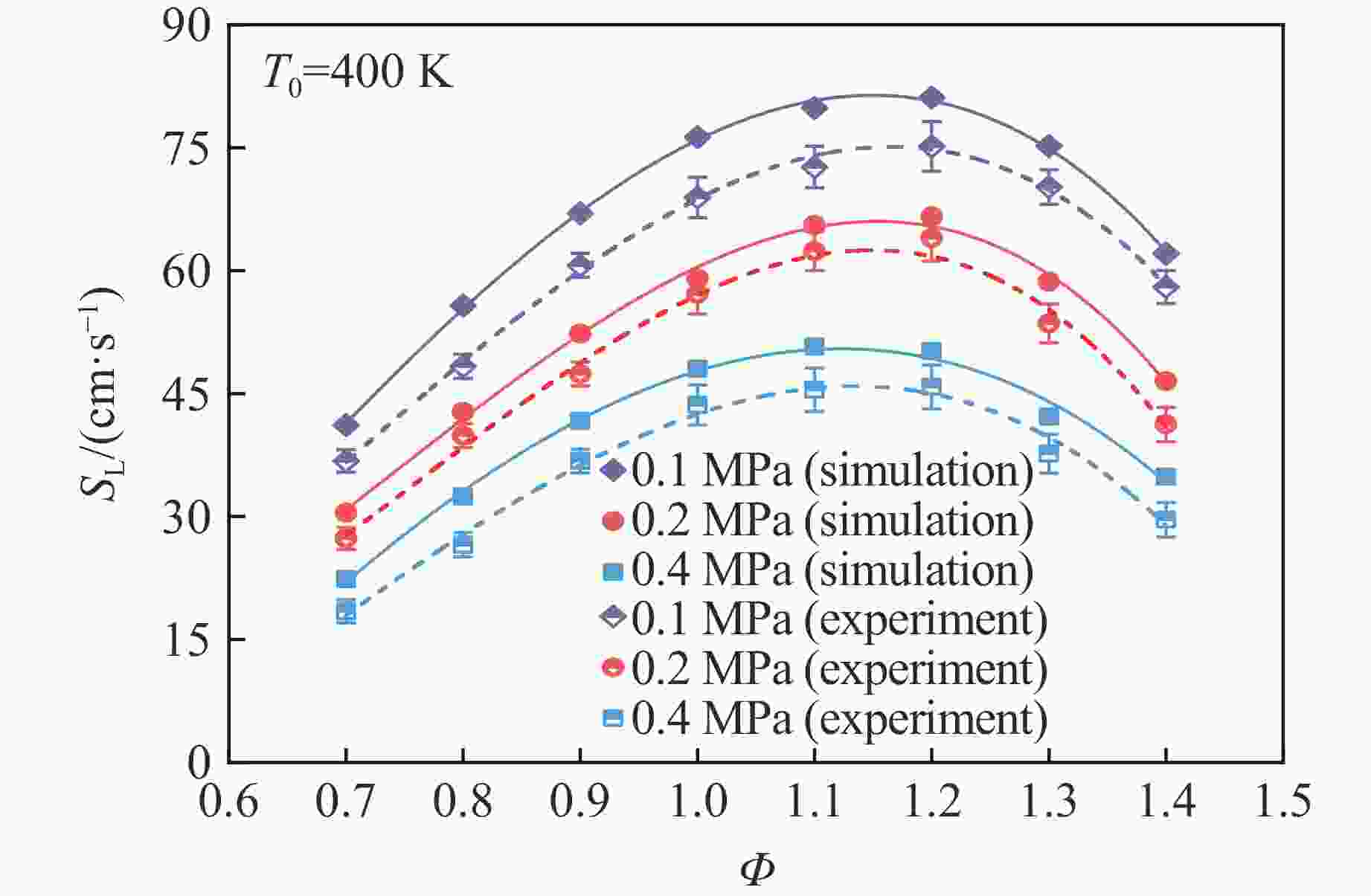
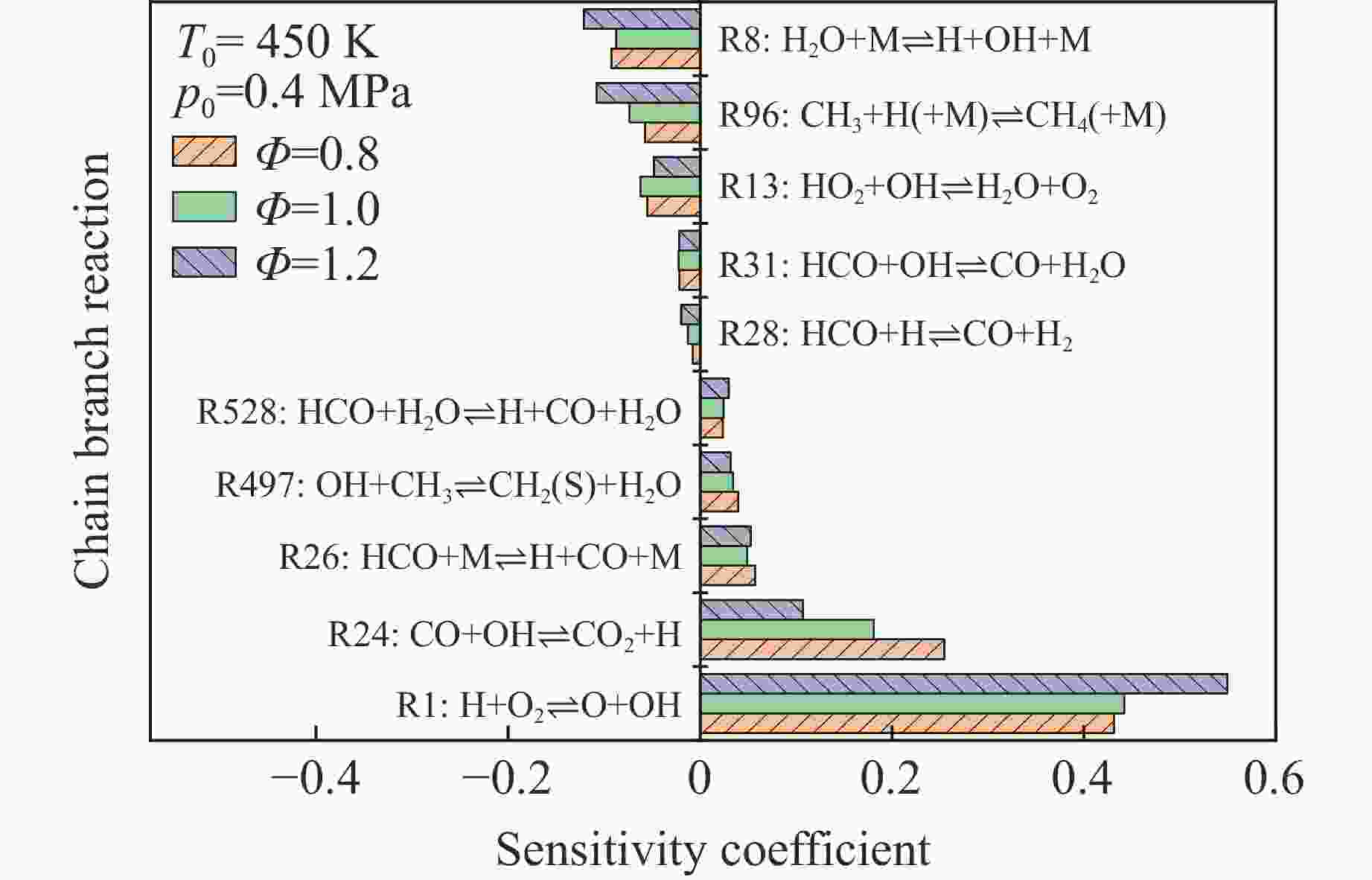
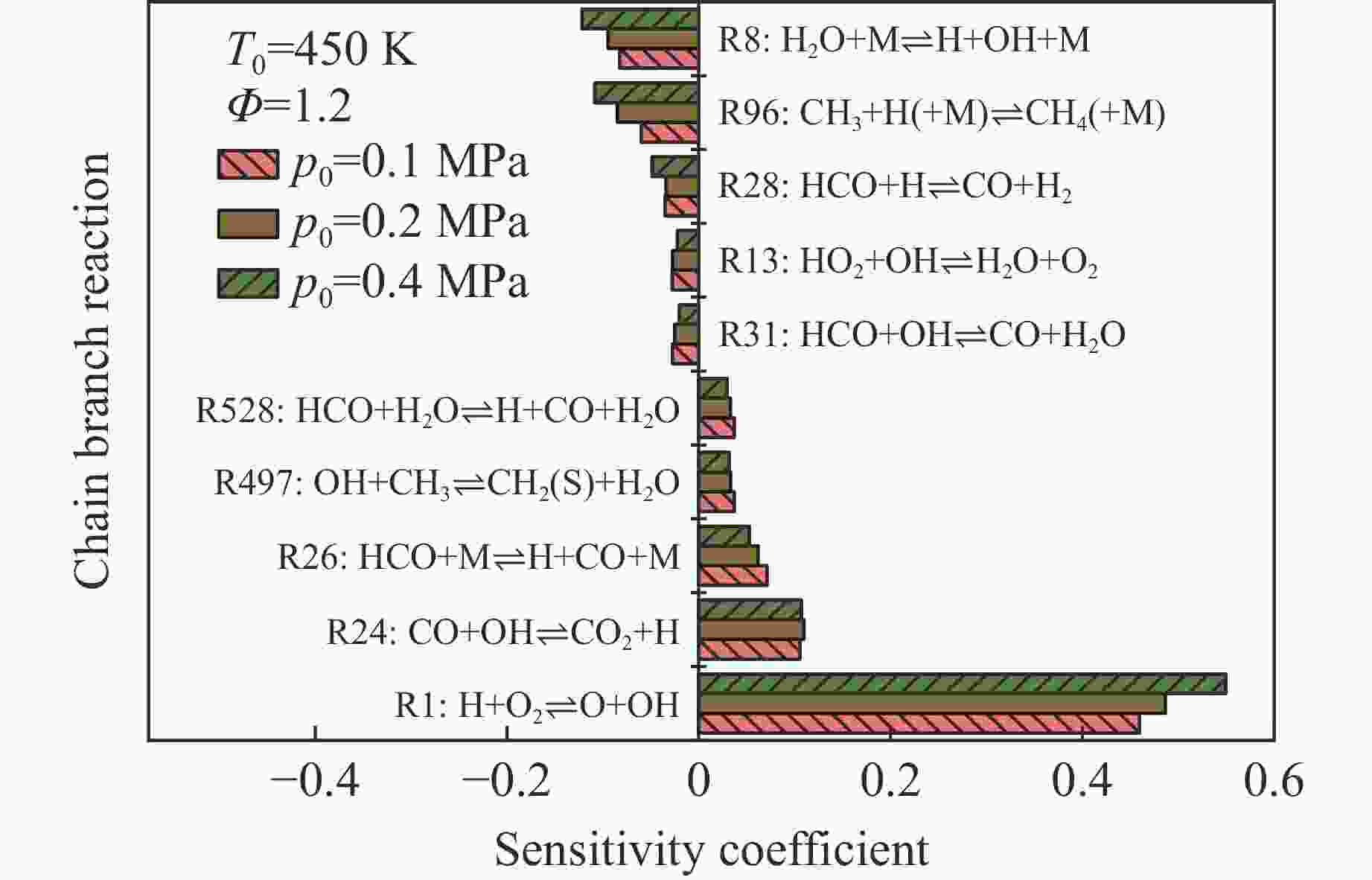
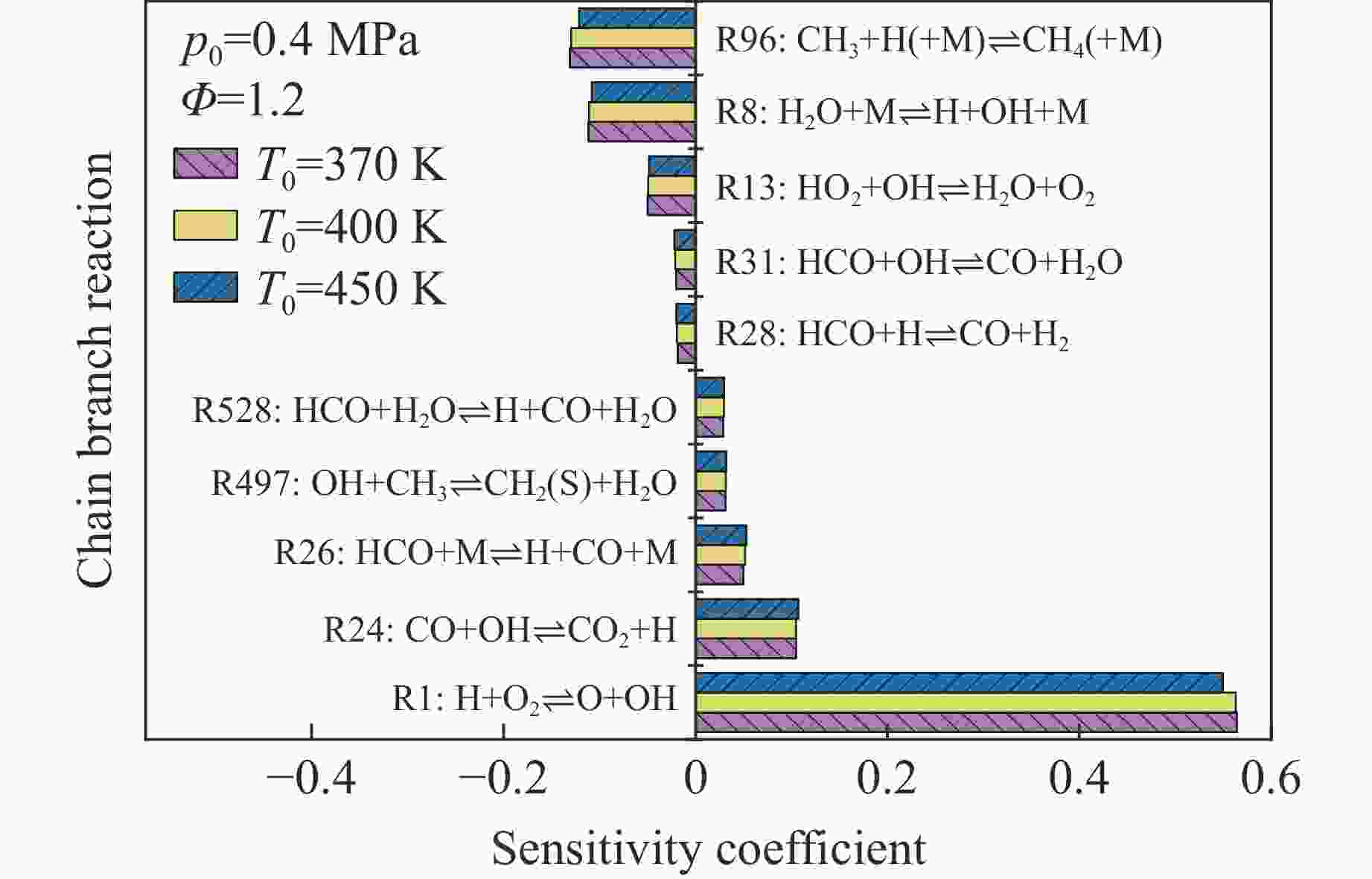
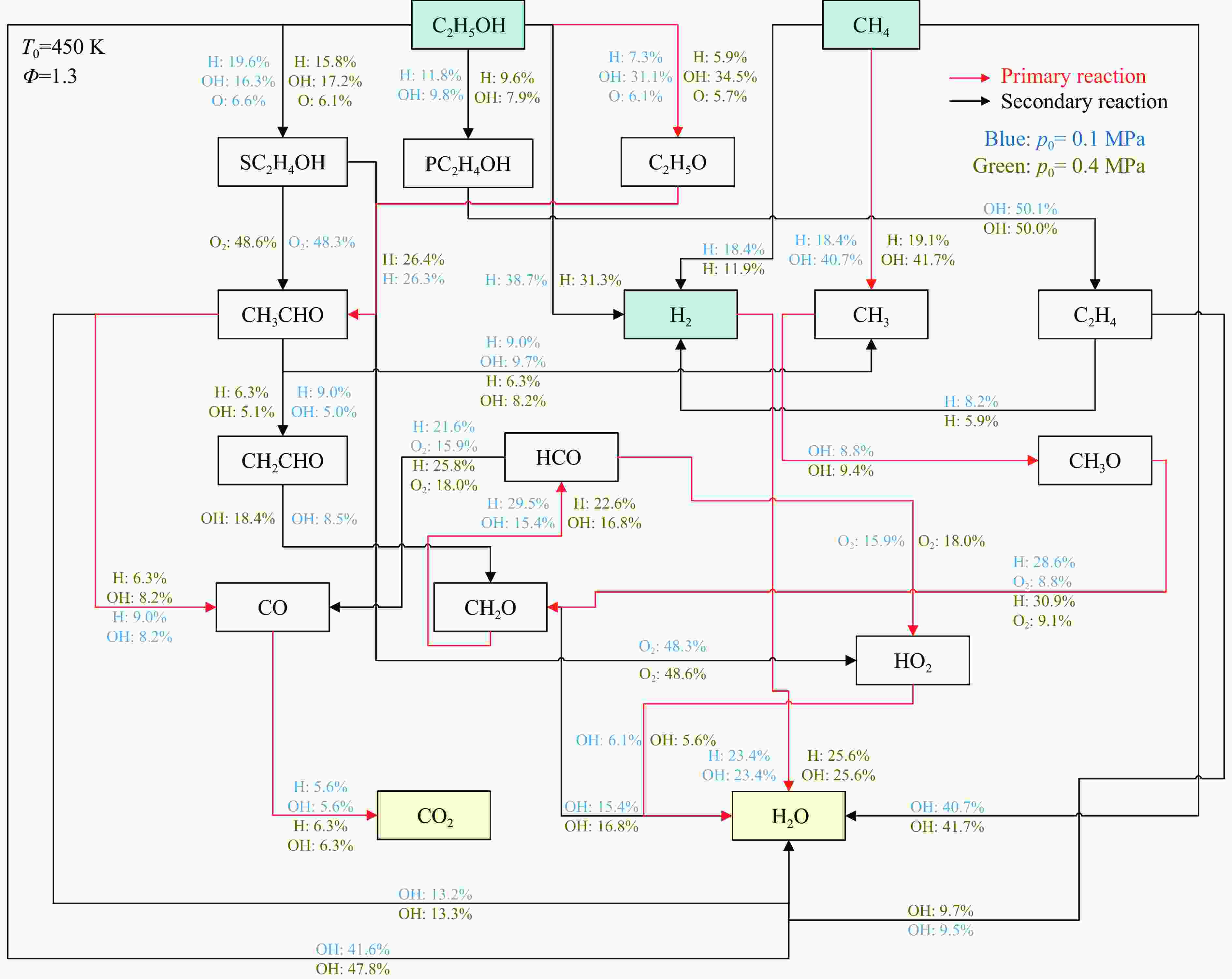
摘要: 乙醇/甲烷/氢气(C2H5OH/CH4/H2)作为一种新型的替代燃料,研究其爆炸特性对于我国新能源的可持续发展具有重要意义。在不同的当量比(0.8~1.4)、初始压力(0.1、0.2和0.4 MPa)和初始温度(370、400和450 K)下,从实验和化学动力学角度分析了其对关键爆炸特性参数,如峰值爆炸压力、峰值爆炸压力上升速率、爆炸时间以及爆燃指数的影响。结果表明,爆炸特性参数在当量比为1.2~1.3之间时出现极值。峰值爆炸压力与初始压力呈线性正相关,而与初始温度呈线性负相关。增大初始压力,火焰锋面裂纹、胞化程度加深,峰值爆炸压力增大。此外,实验工况下评估的最大爆燃指数为20.83 MPa·m/s,表明预混燃料的燃烧处于相对安全水平。基元反应敏感性分析表明:爆燃反应与H和OH自由基密切相关,而R1、R8、R24、R96是影响爆炸反应强度最重要的4个基元反应。研究成果可为C2H5OH/CH4/H2三元混合燃料在实际燃烧装置中的应用、燃料安全性评估以及爆炸事故预防提供参考。Abstract: As a new alternative fuel, ethanol/methane/hydrogen (C2H5OH/CH4/H2) is of great significance for the sustainable development of new energy in China. The effects of different equivalence ratios (0.8−1.4), initial pressures (0.1, 0.2 and 0.4 MPa) and initial temperatures (370, 400 and 450 K) on key explosion characteristics such as peak explosion pressure, peak explosion pressure rise rate, explosion time and deflagration index were analyzed from the experimental and chemical kinetics perspectives. The results show that the explosion characteristic parameters exhibit extreme values when the equivalence ratios between 1.2 and 1.3. The peak explosion pressure is positively correlated with initial pressure and negatively correlated with initial temperature, and this correlation is linear. With the increase in initial pressure, the crack and cytochemical degree of the flame front deepened, and the peak explosion pressure increased. In addition, the maximum deflagration index evaluated under experimental conditions was 20.83 MPa·m/s, indicating that the combustion of premixed fuel/air was at a relatively safe level. The reaction sensitivity analysis of the motives showed that the deflagration reaction was closely related to the H and OH radicals, and R1, R8, R24 and R96 were the top four motif reactions that had the most important impact on the explosion reaction intensity. This work can provide a valuable reference for the application of C2H5OH/CH4/H2 ternary mixed fuel in actual combustion units, the evaluation of fuel safety, and the prevention of explosion accidents.
-
表 1 实验的初始条件
Table 1. Initial conditions of the experiment
Φ p0/MPa T0/K $ {\varphi}_{{\mathrm{C}}_{2}{\mathrm{H}}_{5}\mathrm{O}\mathrm{H}} $/% $ {\varphi}_{{\mathrm{C}\mathrm{H}}_{4}}$/% $ {\varphi}_{{\mathrm{H}}_{2}}$/% 0.8−1.4 0.1, 0.2, 0.4 370, 400, 450 50 40 10 表 2 $T_0 $=450 K下峰值爆炸压力与初始压力的相关性系数
Table 2. Correlation coefficient between peak explosion pressure and initial pressure at $T_0 $=450 K
Φ a b 0.8 −0.039 30 4.716 11 0.9 −0.024 41 5.268 32 1.0 −0.006 71 5.439 43 1.1 −0.020 74 5.788 41 1.2 −0.009 14 5.997 85 表 3 $p_0 $=0.4 MPa下峰值爆炸压力与初始温度的相关性系数
Table 3. Correlation coefficient between peak explosion pressure and initial temperature at $p_0 $=0.4 MPa
Φ c d 0.8 3.409 94 −0.003 45 0.9 3.588 53 −0.003 33 1.0 4.428 85 −0.004 98 1.1 4.585 72 −0.005 12 1.2 4.551 11 −0.004 68 -
[1] LI B L, HANEKLAUS N. The role of clean energy, fossil fuel consumption and trade openness for carbon neutrality in China [J]. Energy Reports, 2022, 8(Suppl 4): 1090–1098. [2] 刘尚, 范钦灏, 王巍, 等. 乙醇汽油点燃压燃模式颗粒物排放特性试验 [J]. 内燃机学报, 2023, 41(1): 33–41.LIU S, FAN Q H, WANG W, et al. Experiment on particulate number emissions of spark ignition to compression ignition combustion mode for ethanol gasoline [J]. Transactions of Csice, 2023, 41(1): 33–41. [3] 刘旭, 钟汶君, 姜鹏, 等. 乙醇汽油/加氢催化生物柴油双燃料发动机着火燃烧及碳烟生成特性研究 [J]. 工程热物理学报, 2022, 43(8): 2143–2151.LIU X, ZHONG W J, JIANG P, et al. Study on ignition combustion and soot formation characteristics of ethanol gasoline-hydrogenated catalytic biodiesel dual fuel engine [J]. Journal of Engineering Thermophysics, 2022, 43(8): 2143–2151. [4] 纪常伟, 辛固, 汪硕峰, 等. 零碳及碳中和燃料内燃机应用进展 [J]. 北京工业大学学报, 2022, 48(3): 273–291. doi: 10.11936/bjutxb2021100007JI C W, XIN G, WANG S F, et al. Application progress of zero carbon and carbon-neutral fuel internal combustion engines [J]. Journal of Beijing University of Technology, 2022, 48(3): 273–291. doi: 10.11936/bjutxb2021100007 [5] WANG X R, ZHANG Y, LI T, et al. Investigation of cellularization characteristics of hydrogen-methane-ethanol expanding spherical flame at elevated pressures [J]. Combustion and Flame, 2023, 255: 112866. doi: 10.1016/j.combustflame.2023.112866 [6] WANG X R, YAN C Z, ZHANG Y, et al. Laminar and kinetic burning characteristics of ethanol/methane/hydrogen fuel: experimental and numerical analysis [J]. Renewable Energy, 2024, 227: 120493. doi: 10.1016/j.renene.2024.120493 [7] LI T, WANG X R, MA Y, et al. Investigation on hydrogen/ethanol intrinsic flame instability [J]. Combustion and Flame, 2022, 241: 112064. doi: 10.1016/j.combustflame.2022.112064 [8] 张衍, 张嘉玮, 王筱蓉. 高压下氢气-乙醇球形膨胀火焰的层流燃烧速度和火焰不稳定性研究 [J]. 新能源进展, 2023, 11(1): 69–75. doi: 10.3969/j.issn.2095-560X.2023.01.010ZHANG Y, ZHANG J W, WANG X R. Investigation of laminar combustion velocity and flame instability in hydrogen-ethanol spherical expansion flames under high pressures [J]. Advances in New and Renewable Energy, 2023, 11(1): 69–75. doi: 10.3969/j.issn.2095-560X.2023.01.010 [9] BAO Y G, LI X L, XU C S, et al. Experimental and numerical study on morphological characteristics and intrinsic instability of premixed hydrogen/ethanol flames [J]. Fuel, 2024, 371: 132019. doi: 10.1016/j.fuel.2024.132019 [10] LI Y C, BI M S, LI B, et al. Effects of hydrogen and initial pressure on flame characteristics and explosion pressure of methane/hydrogen fuels [J]. Fuel, 2018, 233: 269–282. doi: 10.1016/j.fuel.2018.06.042 [11] MOHAMMAD A, JUHANY K A. Laminar burning velocity and flame structure of DME/methane+air mixtures at elevated temperatures [J]. Fuel, 2019, 245: 105–114. doi: 10.1016/j.fuel.2019.02.085 [12] OPPONG F, ZHONGYANG L, LI X L, et al. Investigations on explosion characteristics of ethyl acetate [J]. Journal of Loss Prevention in the Process Industries, 2021, 70: 104409. doi: 10.1016/j.jlp.2021.104409 [13] CHENG J, ZHANG B. Experimental study on the explosion characteristics of ammonia-hydrogen-air mixtures [J]. Fuel, 2024, 363: 131046. doi: 10.1016/j.fuel.2024.131046 [14] MITU M, BRANDES E. Influence of pressure, temperature and vessel volume on explosion characteristics of ethanol/air mixtures in closed spherical vessels [J]. Fuel, 2017, 203: 460–468. doi: 10.1016/j.fuel.2017.04.124 [15] CUI G, WANG S, LIU J G, et al. Explosion characteristics of a methane/air mixture at low initial temperatures [J]. Fuel, 2018, 234: 886–893. doi: 10.1016/j.fuel.2018.07.139 [16] LIU C, TANG K C, HUANG C Y, et al. Effect of initial pressure on the critical characteristics and overpressure of hydrogen-air premixed gas combustion and explosion [J]. International Journal of Hydrogen Energy, 2024, 49: 311–322. doi: 10.1016/j.ijhydene.2023.07.266 [17] SHEN X B, XIU G, WU S Z. Experimental study on the explosion characteristics of methane/air mixtures with hydrogen addition [J]. Applied Thermal Engineering, 2017, 120: 741–747. doi: 10.1016/j.applthermaleng.2017.04.040 [18] 马秋菊, 邵俊程, 王众山, 等. 氢气比例和点火能量对CH4-H2混合气体爆炸强度影响的实验研究 [J]. 高压物理学报, 2020, 34(1): 015201. doi: 10.11858/gywlxb.20190803MA Q J, SHAO J C, WANG Z S, et al. Experimental study of the hydrogen proportion and ignition energy effects on the CH4-H2 mixture explosion intensity [J]. Chinese Journal of High Pressure Physics, 2020, 34(1): 015201. doi: 10.11858/gywlxb.20190803 [19] 张嘉玮, 张衍, 姜根柱, 等. 乙醇-氢气-空气预混燃气爆炸特性的研究 [J]. 新能源进展, 2023, 11(2): 189–196. doi: 10.3969/j.issn.2095-560X.2023.02.012ZHANG J W, ZHANG Y, JIANG G Z, et al. Explosion characteristics of ethanol-hydrogen-air premixed gas [J]. Advances in New and Renewable Energy, 2023, 11(2): 189–196. doi: 10.3969/j.issn.2095-560X.2023.02.012 [20] LIU G L, WANG J, ZHENG L G, et al. Effect of hydrogen addition on explosion characteristics of premixed methane/air mixture under different equivalence ratio distributions [J]. Energy, 2023, 276: 127607. doi: 10.1016/j.energy.2023.127607 [21] LIU L, LUO Z M, SU B, et al. Study on the explosion characteristics and flame propagation of hydrogen-methane-air mixtures in a closed vessel [J]. Journal of Loss Prevention in the Process Industries, 2024, 87: 105224. doi: 10.1016/j.jlp.2023.105224 [22] LIU J J, YU D Y, LI P, et al. Characteristics of explosion hazards in methane-air mixtures diluted by hydrogen [J]. Energies, 2023, 16(18): 6416. doi: 10.3390/en16186416 [23] CHEN Z M, WANG L, ZENG K. A comparative study on the combustion and emissions of dual-fuel engine fueled with natural gas/methanol, natural gas/ethanol, and natural gas/n-butanol [J]. Energy Conversion and Management, 2019, 192: 11–19. doi: 10.1016/j.enconman.2019.04.011 [24] 刘晓龙. 掺氢内燃机燃烧特性及整车燃油经济性的数值模拟研究 [D]. 北京: 北京工业大学, 2015.LIU X L. Numerical investigation on combustion characteristics and vehicle fuel economy performance of hydrogen-enriched engines [D]. Beijing: Beijing University of Technology, 2015. [25] 吕晓辉. 乙醇掺氢燃料预混层流燃烧特性的研究 [D]. 武汉: 武汉理工大学, 2011.LYU X H. Study on premixed laminar combustion of hydrogen blended ethanol fuels [D]. Wuhan: Wuhan University of Technology, 2011. [26] 马熹群. 汽油掺氢层流预混火焰燃烧特性研究[D]. 北京: 北京理工大学, 2016.MA X Q. Study of burning property of gasoline surrogate and hydrogen laminar premixed flame [D]. Beijing: Beijing Institute of Technology, 2016. [27] XU C S, WANG Q Y, SONG Y, et al. Explosion characteristics of n-decane/hydrogen/air mixtures [J]. International Journal of Hydrogen Energy, 2022, 47(91): 38837–38848. doi: 10.1016/j.ijhydene.2022.09.048 [28] LI H Z, XIAO H H. Experimental study on the explosion characteristics of NH3/DME/air mixtures [J]. Fuel, 2023, 352: 129069. doi: 10.1016/j.fuel.2023.129069 [29] OPPONG F, LI X L, XU C S, et al. Investigations on methyl pentanoate-air mixtures confined explosion and cellularity [J]. Fuel, 2024, 358: 130137. doi: 10.1016/j.fuel.2023.130137 [30] OPPONG F, XU C S, LI X L, et al. Laminar flame characteristics of 2-ethylfuran/air mixtures: experimental and kinetic modelling investigations [J]. Fuel, 2022, 307: 121785. doi: 10.1016/j.fuel.2021.121785 [31] HU E J, TIAN H Z, ZHANG X Y, et al. Explosion characteristics of n-butanol/iso-octane-air mixtures [J]. Fuel, 2017, 188: 90–97. doi: 10.1016/j.fuel.2016.10.002 [32] XU C S, WANG H Y, LI X L, et al. Explosion characteristics of a pyrolysis biofuel derived from rice husk [J]. Journal of Hazardous Materials, 2019, 369: 324–333. doi: 10.1016/j.jhazmat.2019.01.101 [33] SAEED K. Determination of the explosion characteristics of methanol-air mixture in a constant volume vessel [J]. Fuel, 2017, 210: 729–737. doi: 10.1016/j.fuel.2017.09.004 [34] MITTAL G, BURKE S M, DAVIES V A, et al. Autoignition of ethanol in a rapid compression machine [J]. Combustion and Flame, 2014, 161(5): 1164–1171. doi: 10.1016/j.combustflame.2013.11.005 [35] OPPONG F, XU C S, ZHONGYANG L, et al. Evaluation of explosion characteristics of 2-methylfuran/air mixture [J]. Journal of Loss Prevention in the Process Industries, 2019, 62: 103954. doi: 10.1016/j.jlp.2019.103954 [36] XIANG L K, JIANG H T, REN F, et al. Numerical study of the physical and chemical effects of hydrogen addition on laminar premixed combustion characteristics of methane and ethane [J]. International Journal of Hydrogen Energy, 2020, 45(39): 20501–20514. doi: 10.1016/j.ijhydene.2019.11.040 [37] DUAN X B, LI Y Y, LIU Y Q, et al. Dilution gas and hydrogen enrichment on the laminar flame speed and flame structure of the methane/air mixture [J]. Fuel, 2020, 281: 118794. doi: 10.1016/j.fuel.2020.118794 [38] ECKART S, PIZZUTI L, FRITSCHE C, et al. Experimental study and proposed power correlation for laminar burning velocity of hydrogen-diluted methane with respect to pressure and temperature variation [J]. International Journal of Hydrogen Energy, 2022, 47(9): 6334–6348. doi: 10.1016/j.ijhydene.2021.11.243 [39] BERWAL P, SOLAGAR S, KUMAR S. Experimental investigations on laminar burning velocity variation of CH4+H2+air mixtures at elevated temperatures [J]. International Journal of Hydrogen Energy, 2022, 47(37): 16686–16697. doi: 10.1016/j.ijhydene.2022.03.155 [40] ARAVINDAN M, PRAVEEN KUMAR G, ARULANANDAM M K, et al. Multi-objective optimization and analysis of chemical kinetics properties: exploring the impact of different hydrogen blending ratios on LPG and methane-air mixtures [J]. Energy Conversion and Management: X, 2024, 22: 100532. doi: 10.1016/j.ecmx.2024.100532 [41] MORSY M E, YANG J F. The instability of laminar methane/hydrogen/air flames: correlation between small and large-scale explosions [J]. International Journal of Hydrogen Energy, 2022, 47(69): 29959–29970. doi: 10.1016/j.ijhydene.2022.06.289 [42] 李祥春, 聂百胜, 杨春丽, 等. 封闭空间内瓦斯浓度对瓦斯爆炸反应动力学特性的影响 [J]. 高压物理学报, 2017, 31(2): 135–147. doi: 10.11858/gywlxb.2017.02.005LI X C, NIE B S, YANG C L, et al. Effect of gas concentration on kinetic characteristics of gas explosion in confined space [J]. Chinese Journal of High Pressure Physics, 2017, 31(2): 135–147. doi: 10.11858/gywlxb.2017.02.005 [43] 杨春丽, 刘艳, 胡玢, 等. 氮气和水蒸气对瓦斯爆炸基元反应的影响及抑爆机理分析 [J]. 高压物理学报, 2017, 31(3): 301–308. doi: 10.11858/gywlxb.2017.03.012YANG C L, LIU Y, HU F, et al. Effect of nitrogen and water vapor on methane-air mixture explosion elementary reaction and suppression mechanism [J]. Chinese Journal of High Pressure Physics, 2017, 31(3): 301–308. doi: 10.11858/gywlxb.2017.03.012 [44] 夏煜, 程扬帆, 胡芳芳, 等. 典型固体抑爆剂对乙炔-空气的抑爆特性 [J]. 高压物理学报, 2022, 36(6): 065201. doi: 10.11858/gywlxb.20220580XIA Y, CHENG Y F, HU F F, et al. Inhibition characteristics of typical solid explosion suppressors on acetylene-air explosion [J]. Chinese Journal of High Pressure Physics, 2022, 36(6): 065201. doi: 10.11858/gywlxb.20220580 -






 下载:
下载: