Structural Stability and Shock Decomposition of UH3 at High Temperature and High Pressure
-
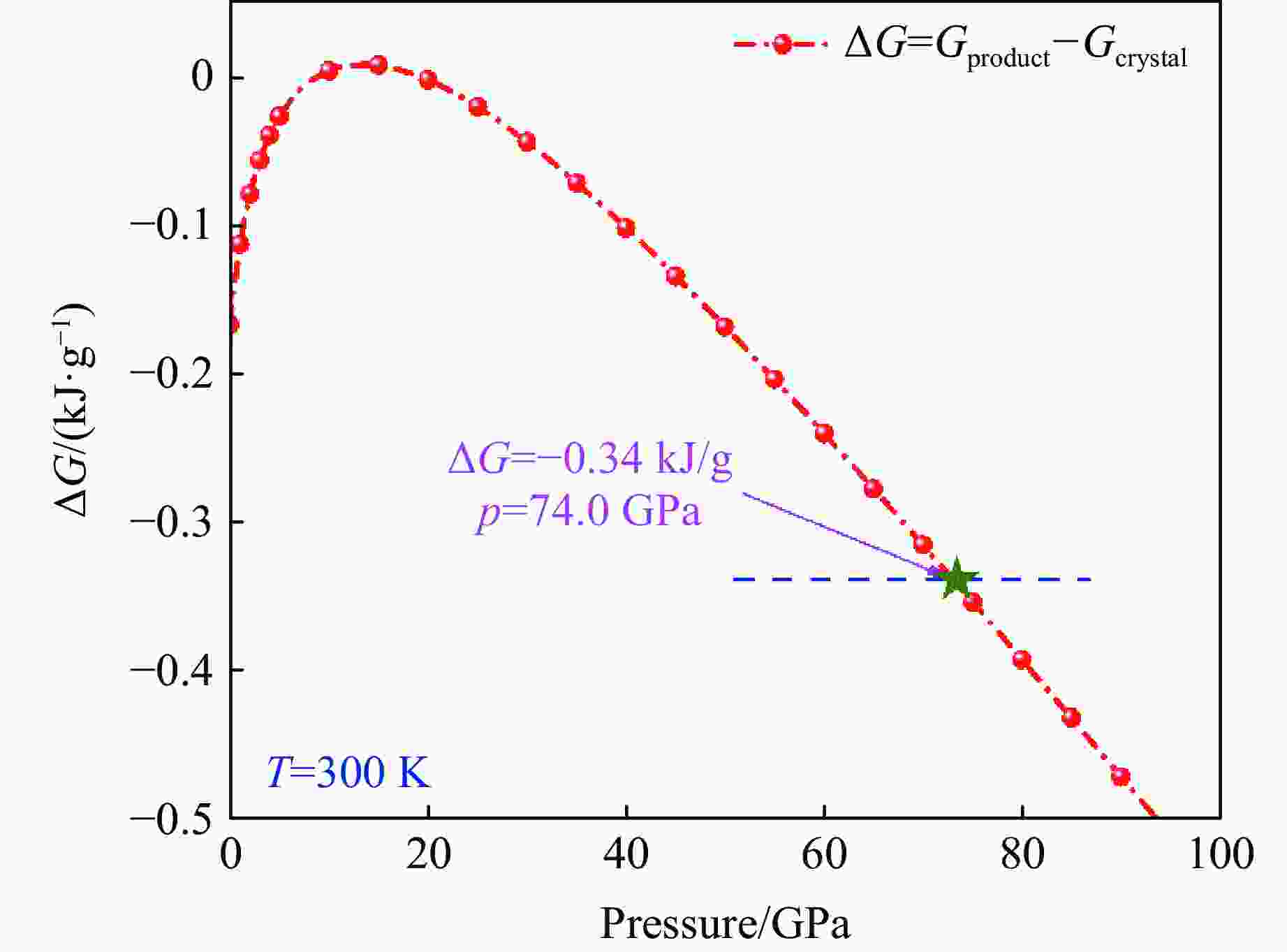
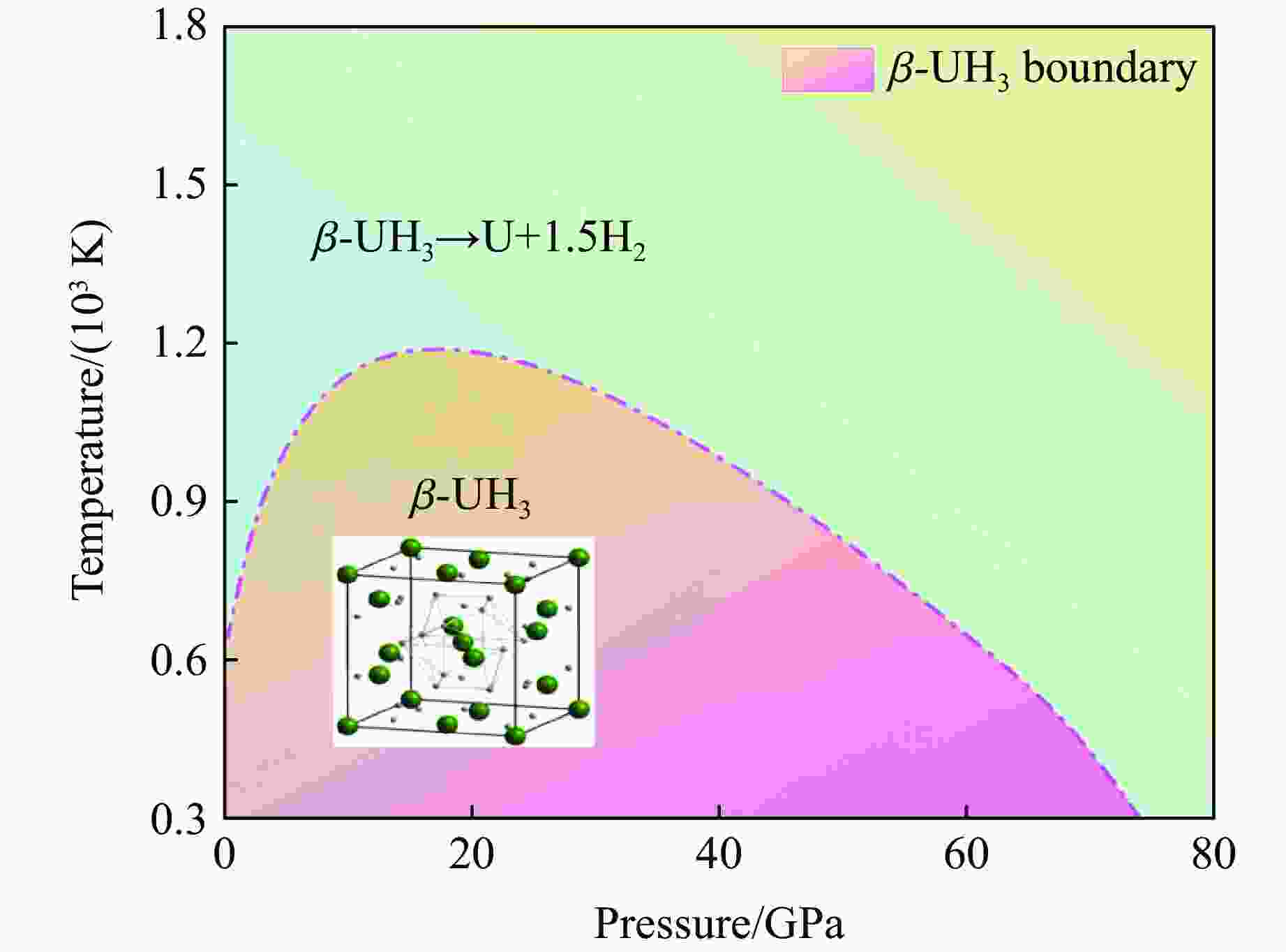
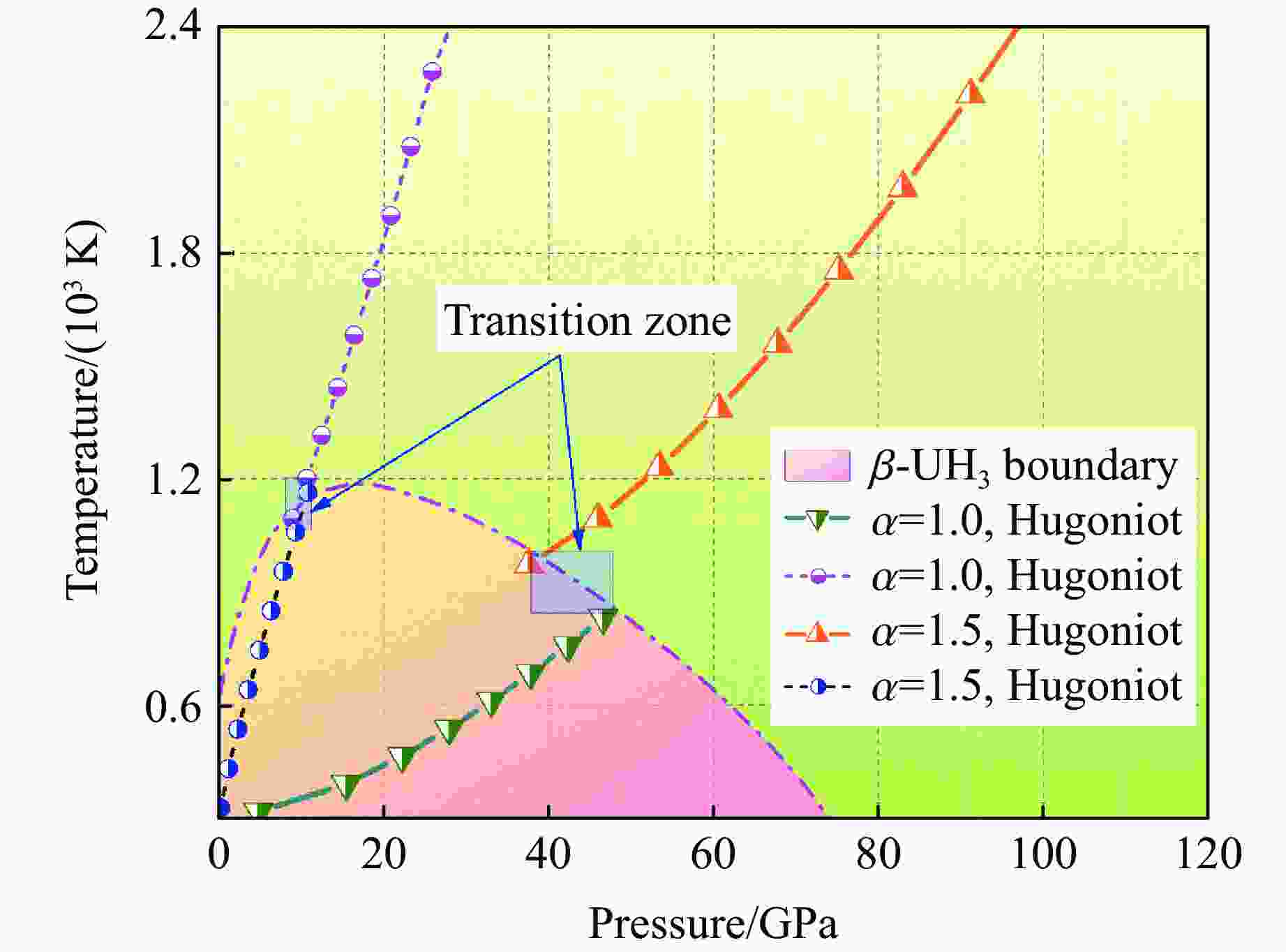
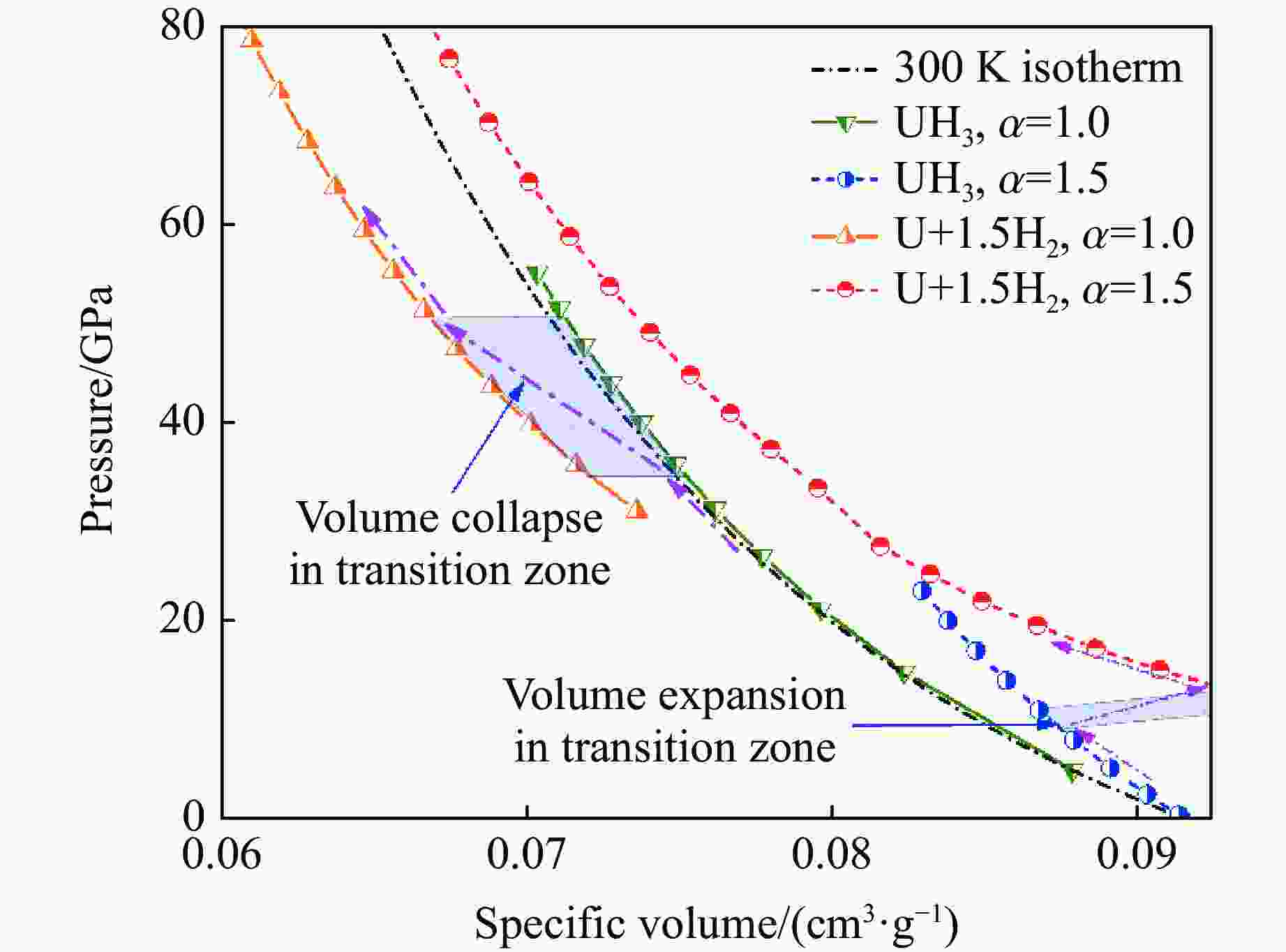
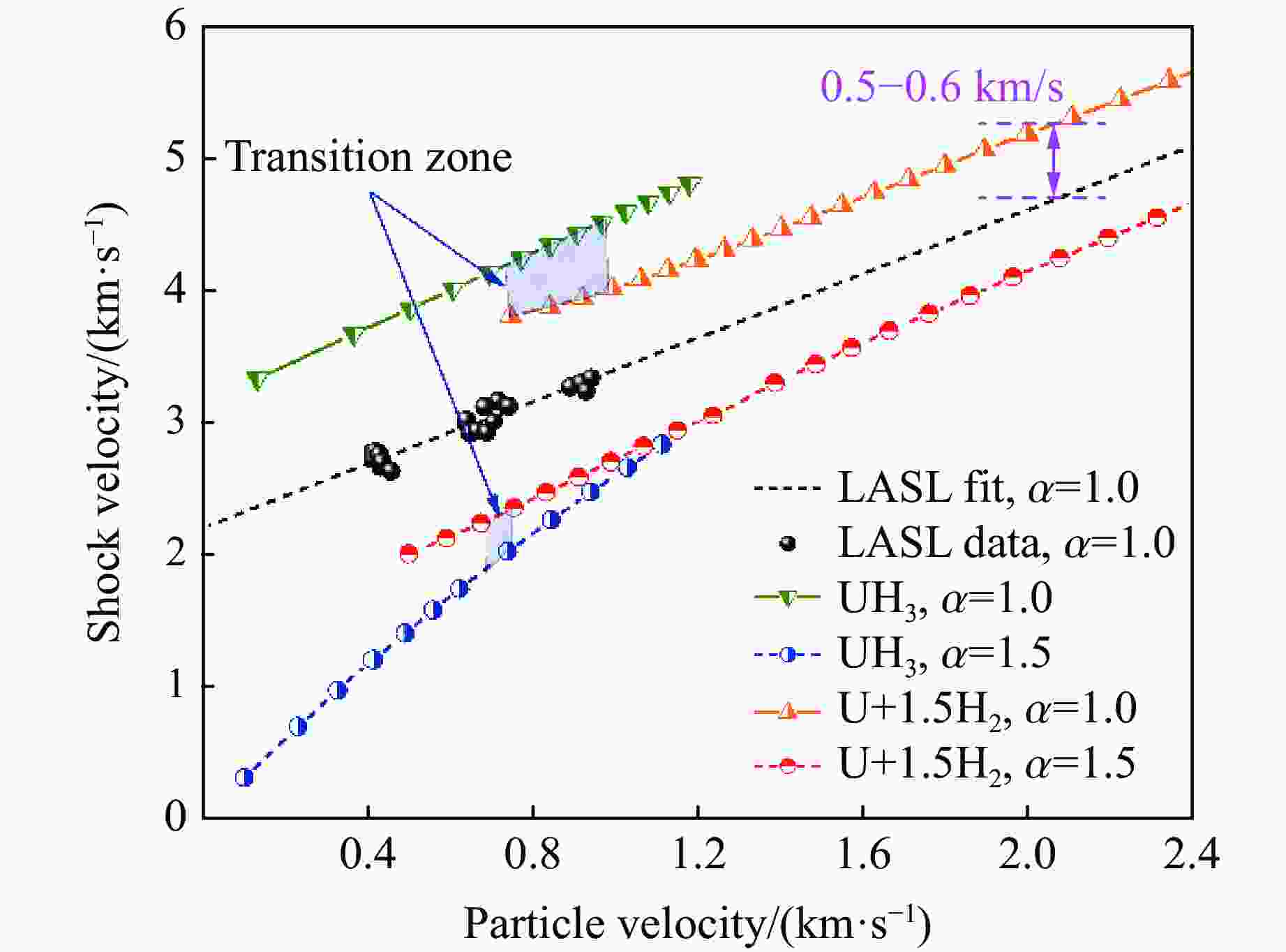
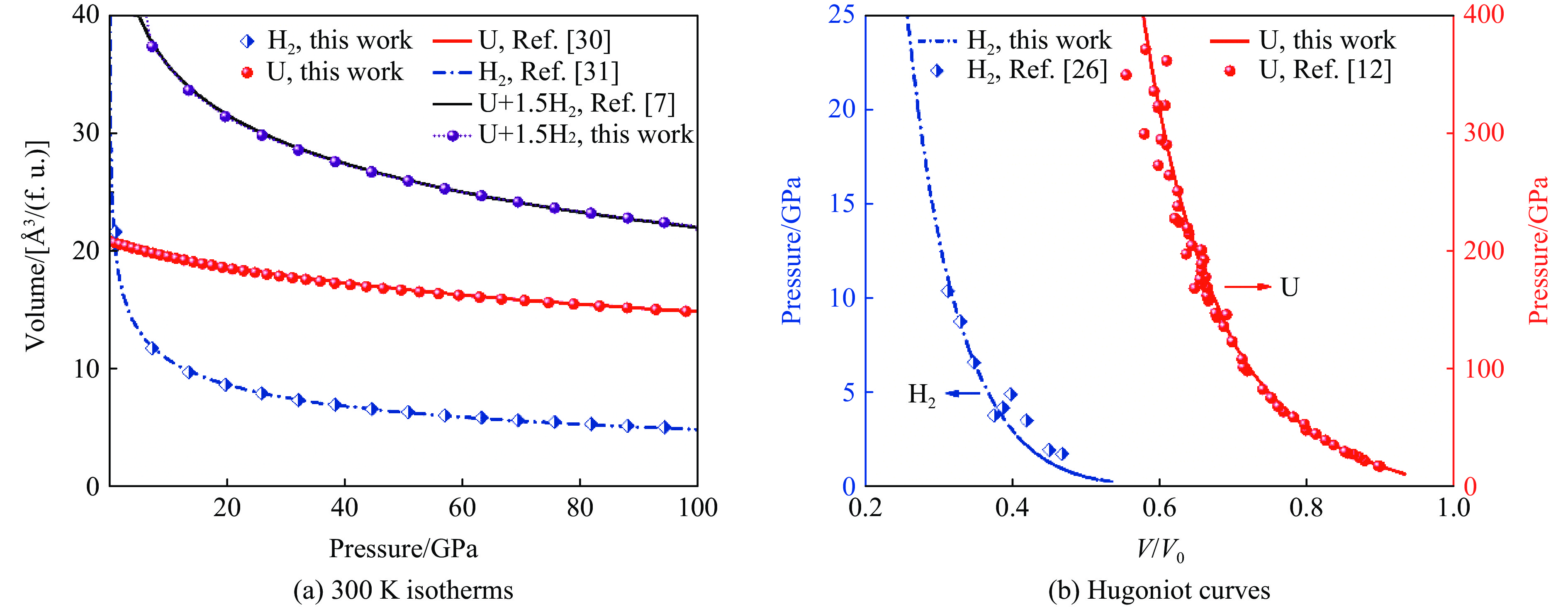
摘要: 利用统计物理模型构建了UH3晶体及其化学分解产物的状态方程,通过比较Gibbs自由能获得了UH3的高温高压相图,并将其应用于疏松和密实UH3冲击压缩性质研究中。结果表明:等温压缩下,UH3晶体在压力约74.0 GPa时发生化学分解,提高温度有助于化学分解的发生,但压力对UH3化学分解相边界的影响是非单调的;冲击加载下,密实UH3在35~50 GPa压力范围内发生化学分解,并且由于冲击分解伴随着明显的体积塌缩,分解产物的雨贡纽曲线位于等温压缩线下方,曲线位置关系反常;UH3的冲击分解压力随着疏松度的增大而减小,当UH3材料的初始疏松度为1.5时,在化学分解转变压力范围内,UH3的分解产物比UH3晶体更难压缩,表现出类似大疏松度材料在冲击作用下的“反常膨胀”现象。研究结果丰富了对UH3材料动态压缩特性的认识,为锕系金属氢化物的高温高压物理化学性质研究提供了理论参考。Abstract: Using statistical physical model, the equation of state of UH3 crystal and its chemical decomposition products were constructed in this paper. The phase diagram of UH3 at high temperature and high pressure was obtained by Gibbs free energy comparison, and the shock compression properties of UH3 with different initial densities were investigated. The results show that the chemical decomposition of UH3 crystals occurs at about 74.0 GPa under isothermal compression. Increasing the temperature promotes the chemical decomposition, but the influence of pressure on the chemical decomposition of UH3 is non-monotonic. Solid UH3 decomposes at 35–50 GPa under shock compression, and the chemical decomposition process is accompanied by obvious volume collapse, therefore, the Hugoniot of UH3 decomposition products lies below the isotherm, which is an abnormal phenomenon in comparison with ordinary metals or compounds. Moreover, the decomposition pressure of UH3 decreases with the increase of initial porosity. When the initial porosity is about 1.5, the decomposition products of UH3 are more difficult to compress than UH3 in crystal phase, thus showing a phenomenon similar to the abnormal expansion of large porosity materials under shock compression. These results enrich our understanding of dynamical compression behavior of UH3, and can serve as theoretical basis for further research on physical and chemical properties of actinide metal hydrides at high temperature and high pressure.
-
Key words:
- UH3 /
- hydride /
- equation of state /
- high temperature and high pressure /
- structural stability /
- chemical decomposition
-
表 1 UH3晶体相的状态方程参数
Table 1. Equation of state parameters for UH3 crystal phase
V0/(cm3·g−1) V0K/(cm3·g−1) B0/GPa $ {B}'{_{{ 0}}}$ ΘD0/K ΘE0/K g1 g2 g3 g4 0.0916 0.0882 123 4.76 169 1400 −0.1 1.2 4.17 −3.23 -
[1] SOUTER P F, KUSHTO G P, ANDREWS L, et al. Experimental and theoretical evidence for the formation of several uranium hydride molecules [J]. Journal of the American Chemical Society, 1997, 119(7): 1682–1687. doi: 10.1021/ja9630809 [2] BANOS A, HARKER N J, SCOTT T B. A review of uranium corrosion by hydrogen and the formation of uranium hydride [J]. Corrosion Science, 2018, 136: 129–147. doi: 10.1016/j.corsci.2018.03.002 [3] LE GUYADEC F, GÉNIN X, BAYLE J P, et al. Pyrophoric behaviour of uranium hydride and uranium powders [J]. Journal of Nuclear Materials, 2010, 396(2/3): 294–302. doi: 10.1016/j.jnucmat.2009.11.007 [4] BLOCH J, MINTZ M H. Kinetics and mechanisms of metal hydrides formation: a review [J]. Journal of Alloys and Compounds, 1997, 253/254: 529–541. doi: 10.1016/S0925-8388(96)03070-8 [5] DROZDOV A P, KONG P P, MINKOV V S, et al. Superconductivity at 250 K in lanthanum hydride under high pressures [J]. Nature, 2019, 569(7757): 528–531. doi: 10.1038/s41586-019-1201-8 [6] KONG P P, MINKOV V S, KUZOVNIKOV M A, et al. Superconductivity up to 243 K in the yttrium-hydrogen system under high pressure [J]. Nature Communications, 2021, 12(1): 5075. doi: 10.1038/s41467-021-25372-2 [7] KRUGLOV I A, KVASHNIN A G, GONCHAROV A F, et al. Uranium polyhydrides at moderate pressures: prediction, synthesis, and expected superconductivity [J]. Science Advances, 2018, 4(10): eaat9776. doi: 10.1126/sciadv.aat9776 [8] GUIGUE B, MARIZY A, LOUBEYRE P. Synthesis of UH7 and UH8 superhydrides: additive-volume alloys of uranium and atomic metal hydrogen down to 35 GPa [J]. Physical Review B, 2020, 102(1): 014107. doi: 10.1103/PhysRevB.102.014107 [9] KÝVALA L, HAVELA L, KADZIELAWA A P, et al. Electrons and phonons in uranium hydrides: effects of polar bonding [J]. Journal of Nuclear Materials, 2022, 567: 153817. doi: 10.1016/j.jnucmat.2022.153817 [10] WANG X H, LI M L, ZHENG F W, et al. Crystal structure prediction of uranium hydrides at high pressure: a new hydrogen-rich phase [J]. Physics Letters A, 2018, 382(40): 2959–2964. doi: 10.1016/j.physleta.2018.06.040 [11] LIU M, SHI Y P, LIU M F, et al. First-principles comprehensive study of electronic and mechanical properties of novel uranium hydrides at different pressures [J]. Progress in Natural Science: Materials International, 2020, 30(2): 251–259. doi: 10.1016/j.pnsc.2020.01.019 [12] MARSH S P. LASL shock Hugoniot data [M]. Berkeley: University of California Press, 1980. [13] SYONO Y, KUSABA K, FUKUOKA K, et al. Shock compression of V2H and V2D to 135 GPa and anomalous decompression behavior [J]. Physical Review B, 1984, 29(12): 6520–6524. doi: 10.1103/PhysRevB.29.6520 [14] TAGUCHI H, FUKAI Y, ATOU T, et al. Shock compression of NbH0.75 and TaH0.50: universal compression behavior of hydrogen in metallic environments [J]. Physical Review B, 1994, 49(5): 3025–3029. doi: 10.1103/physrevb.49.3025 [15] GOLUBKOV A N, GUDARENKO L F, ZHERNOKLETOV M V, et al. Shock compression of vanadium hydrides and deuterides with different concentrations of gas atoms [J]. Combustion, Explosion, and Shock Waves, 2017, 53(3): 309–318. doi: 10.1134/S001050821703008X [16] GOLUBKOV A N, GUDARENKO L F, ZHERNOKLETOV M V, et al. Shock compression of titanium hydride and titanium, tantalum, and zirconium deuterides [J]. Combustion, Explosion, and Shock Waves, 2021, 57(4): 479–486. doi: 10.1134/S0010508221040110 [17] TAYLOR C D, LOOKMAN T, LILLARD R S. Ab initio calculations of the uranium-hydrogen system: thermodynamics, hydrogen saturation of α-U and phase-transformation to UH3 [J]. Acta Materialia, 2010, 58(3): 1045–1055. doi: 10.1016/j.actamat.2009.10.021 [18] FILANOVICH A N, POVZNER A A. Modeling of unusual lattice properties of superconducting PuCoIn5 based on ab initio calculation [J]. Physica B: Condensed Matter, 2019, 575: 411693. doi: 10.1016/j.physb.2019.411693 [19] SJOSTROM T, CROCKETT S, RUDIN S. Multiphase aluminum equations of state via density functional theory [J]. Physical Review B, 2016, 94(14): 144101. doi: 10.1103/PhysRevB.94.144101 [20] 吴强, 经福谦, 李欣竹. 零温物态方程输入参数B0K、 ${B'_{{\mathrm{0K}}} }$ 和ρ0K的确定 [J]. 高压物理学报, 2005, 19(2): 97–104. doi: 10.11858/gywlxb.2005.02.001WU Q, JING F Q, LI X Z. Determination of the input parameters B0K、${B'_{{\mathrm{0K}}} }$ 、ρ0K for 0 K universal isothermal equation of state [J]. Chinese Journal of High Pressure Physics, 2005, 19(2): 97–104. doi: 10.11858/gywlxb.2005.02.001[21] OLSSON P A T, BLOMQVIST J, BJERKÉN C, et al. Ab initio thermodynamics investigation of titanium hydrides [J]. Computational Materials Science, 2015, 97: 263–275. doi: 10.1016/j.commatsci.2014.10.029 [22] NEKRASOV I, OVCHINIKOV S. Hydrides under high pressure [J]. Journal of Superconductivity and Novel Magnetism, 2022, 35(4): 959–963. doi: 10.1007/s10948-021-06087-3 [23] SALKE N P, ESFAHANI M M D, ZHANG Y J, et al. Synthesis of clathrate cerium superhydride CeH9 at 80–100 GPa with atomic hydrogen sublattice [J]. Nature Communications, 2019, 10(1): 4453. doi: 10.1038/s41467-019-12326-y [24] ZHANG L, ZHAO Y H, SONG H Z, et al. Initial decomposition mechanisms and the inverse effects of temperature and PH2 on the thermodynamics stability of UH3 [J]. Physical Chemistry Chemical Physics, 2023, 23(17): 12515–12521. doi: 10.1039/D2CP05931B [25] GROVER R. Liquid metal equation of state based on scaling [J]. The Journal of Chemical Physics, 1971, 55(7): 3435–3441. doi: 10.1063/1.1676596 [26] ROSS M, REE F H, YOUNG D A. The equation of state of molecular hydrogen at very high density [J]. The Journal of Chemical Physics, 1983, 79(3): 1487–1494. doi: 10.1063/1.445939 [27] ZHANG T T, WANG Y C, XIAN J W, et al. Effect of the projector augmented wave potentials on the simulation of thermodynamic properties of vanadium [J]. Matter and Radiation at Extremes, 2021, 6(6): 068401. doi: 10.1063/5.0059360 [28] LIU H F, SONG H F, ZHANG Q L, et al. Validation for equation of state in wide regime: copper as prototype [J]. Matter and Radiation at Extremes, 2016, 1(2): 123–131. doi: 10.1016/j.mre.2016.03.002 [29] LI Q, LIU H F, ZHANG G M, et al. The thermodynamical instability induced by pressure ionization in fluid helium [J]. Physics of Plasmas, 2016, 23(11): 112709. doi: 10.1063/1.4968828 [30] DEWAELE A, BOUCHET J, OCCELLI F, et al. Refinement of the equation of state of α-uranium [J]. Physical Review B, 2013, 88(13): 134202. doi: 10.1103/PhysRevB.88.134202 [31] JI C, LI B, LIU W J, et al. Ultrahigh-pressure isostructural electronic transitions in hydrogen [J]. Nature, 2019, 573(7775): 558–562. doi: 10.1038/s41586-019-1565-9 [32] YOO C S, AKELLA J, MORIARTY J A. High-pressure melting temperatures of uranium: laser-heating experiments and theoretical calculations [J]. Physical Review B, 1993, 48(21): 15529–15534. doi: 10.1103/physrevb.48.15529 [33] MINEEV V N, FUNTIKOV A I. Measurements of the viscosity of iron and uranium under shock compression [J]. High Temperature, 2006, 44(6): 941–949. doi: 10.1007/s10740-006-0113-0 [34] PANKRATOV D G, YAKUNIN A K, POPTSOV A G, et al. Sound velocity in natural shock-compressed uranium in a pressure range of 20–260 GPa [J]. Combustion, Explosion, and Shock Waves, 2021, 57(6): 746–750. doi: 10.1134/S0010508221060149 [35] DUFFY T S, VOS W L, ZHA C S, et al. Sound velocities in dense hydrogen and the interior of Jupiter [J]. Science, 1994, 263(5153): 1590–1593. doi: 10.1126/science.263.5153.1590 [36] FREIMAN Y A, GRECHNEV A, TRETYAK S M, et al. Sound velocities in solid hydrogen under pressure [J]. Low Temperature Physics, 2013, 39(5): 423–426. doi: 10.1063/1.4807043 [37] WU J F, WANG Y C, LIU Y, et al. First-principles study on the electronic structure transition of β-UH3 under high pressure [J]. Matter and Radiation at Extremes, 2022, 7(5): 058402. doi: 10.1063/5.0091969 [38] ABRAHAM B M, FLOTOW H E. The heats of formation of uranium hydride, uranium deuteride and uranium tritide at 25 ℃ [J]. Journal of the American Chemical Society, 1955, 77(6): 1446–1448. doi: 10.1021/ja01611a013 [39] GENG H Y, WU Q, TAN H, et al. Extension of the Wu-Jing equation of state for highly porous materials: thermoelectron based theoretical model [J]. Journal of Applied Physics, 2002, 92(10): 5924–5929. doi: 10.1063/1.1516619 [40] GENG H Y, WU Q, TAN H, et al. Extension of the Wu-Jing equation of state for highly porous materials: calculations to validate and compare the thermoelectron model [J]. Journal of Applied Physics, 2002, 92(10): 5917–5923. doi: 10.1063/1.1516618 [41] KENT P R C, KOTLIAR G. Toward a predictive theory of correlated materials [J]. Science, 2018, 361(6400): 348–354. doi: 10.1126/science.aat5975 -






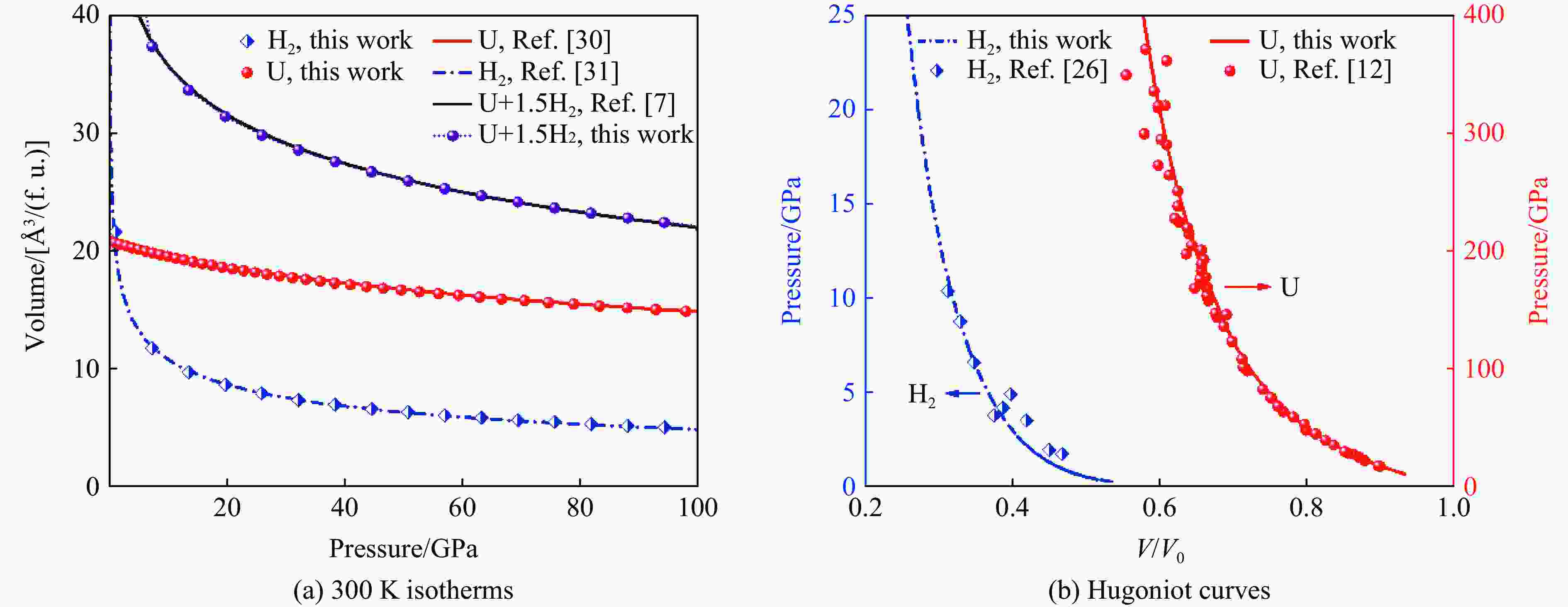
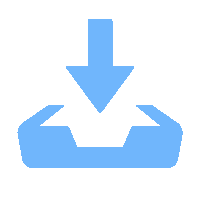 下载:
下载: