High-Pressure Solid-State Metathesis Synthesis ofTernary Iron-Based Metal Nitrides
-
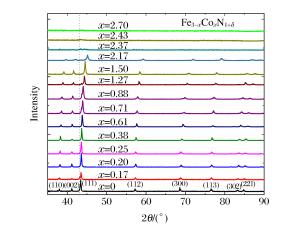
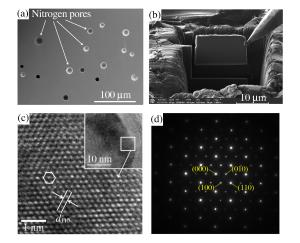
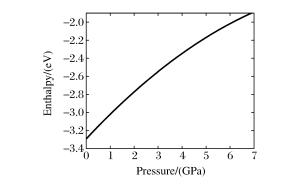
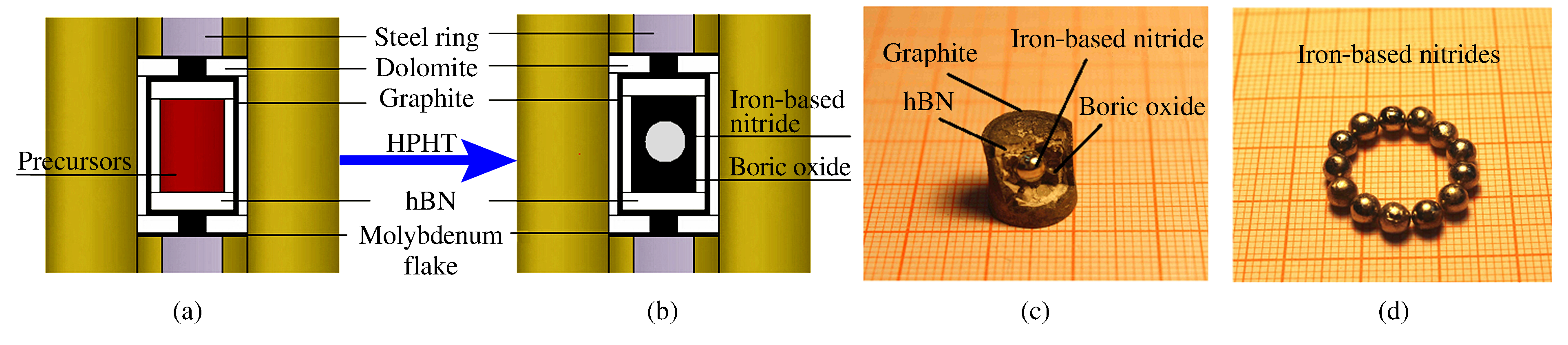
摘要: 以二元金属氧化物(氧化铁、氧化钴、氧化镍)和六方氮化硼为反应前驱体,在大腔体压机提供的高温高压条件下(5GPa、1673K),通过发生复合高压固相复分解(HPSSM)反应合成组分可调控的三元铁基金属氮化物圆球状块体材料ε-Fe3-xMxN1+δ(M=Co, Ni)。并利用X射线粉末衍射(XPD)、场发射扫描电子显微镜(FE-SEM)、高分辨率透射电子显微镜(HRTEM)等多种材料表征手段对高压合成的三元铁基金属氮化物进行结构表征, 同时基于密度泛函理论(DFT)的第一性原理计算探究压力对HPSSM反应的影响。研究结果表明,高压密闭环境有利于制备高质量金属氮化物,HPSSM反应合成法是制备铁基金属氮化物块体材料的一种有效方法。Abstract: Ternary ε-Fe3-xMxN1+δ (M=Co, Ni) were synthesized as spherical bulk materials with variable components through composite high-pressure solid-state metathesis (HPSSM) reactions under 5GPa and at 1673K, employing diversity binary metal oxides (Fe2O3, CoO, NiO) and hBN as the reaction precursors.The structural characterizations of the as-prepared iron-based metal nitrides were determined by X-ray powder diffraction (XPD), field-emission scanning electronic microscopy (FE-SEM) and high-resolution transmission electron microscope (HRTEM), etc.First-principles calculations were used to explore the effect of pressure on the reaction enthalpy ΔH in HPSSM.Our results show that the high-pressure confinement environment is favorable for the synthesis of high-quality metal nitrides, and the HPSSM reaction is an effective synthetic route to the bulk iron-based metal nitrides.
-
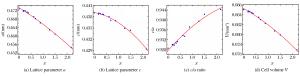
表 1 P6322空间群构型的铁基金属氮化物精修晶格参数
Table 1. Crystal structure data of iron-based metal nitrides for refinements in space group P6322
Iron-based nitrides a/(nm) c/(nm) V/(nm3) ε-Fe3N1.27 0.47413(5) 0.43993(5) 0.085645(34) ε-Fe2.67Co0.33N0.99 0.47132(3) 0.43862(7) 0.084380(34) ε-Fe2.32Co0.68N0.98 0.46824(9) 0.43705(14) 0.082988(74) ε-Fe2.67Ni0.33N0.86 0.47004(4) 0.43846(5) 0.083893(29) ε-Fe2.29Ni0.71N0.79 0.47380(4) 0.43853(6) 0.085257(34) -
[1] GRABKE H J.The role of nitrogen in the corrosion of iron and steels[J].ISIJ Int, 1996, 36(7):777-786. doi: 10.2355/isijinternational.36.777 [2] WANG X, ZHENG W T, TIAN H W, et al.Growth, structural, and magnetic properties of iron nitride thin films deposited by dc magnetron sputtering[J].Appl Surf Sci, 2003, 220(1):30-39. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fee3eab003be0e1f53d2b115a566f564 [3] MORISAKO A, MATSUMOTO M, NAOE M.Magnetic anisotropy and soft magnetism of iron nitride thin films prepared by facing-target sputtering[J].J Appl Phys, 1991, 69(8):5619-5621. doi: 10.1063/1.347941 [4] NAGAKURA S, TANEHASHI K.Electronic structure of iron nitrides studied by electron diffraction.Ⅱ.ε-Fe2N and ζ-Fe2N[J].J Phys Soc Jpn, 1968, 25(3):840-846. doi: 10.1143/JPSJ.25.840 [5] PANDA R N, GAJBHIYE N S.Magnetic properties of single domain ε-Fe3N synthesized by borohydride reduction route[J].J Appl Phys, 1997, 81(1):335-339. doi: 10.1063/1.364115 [6] ATIQ S, KO H, SIDDIQI S A, et al.Effect of epitaxy and lattice mismatch on saturation magnetization of γ'-Fe4N thin films[J].Appl Phys Lett, 2008, 92(22):222-507. [7] DIRBA I, KOMISSINSKIY P, GUTFLEISCH O, et al.Increased magnetic moment induced by lattice expansion from α-Fe to α'-Fe8N[J].J Appl Phys, 2015, 117(17):173-911. doi: 10.1063/1.4919601 [8] SIFKOVITS M, SMOLINSKI H, HELLWIG S, et al.Interplay of chemical bonding and magnetism in Fe4N, Fe3N and ζ-Fe2N[J].J Magn Magn Mater, 1999, 204(3):191-198. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ027170675/ [9] LEINEWEBER A, JACOBS H, HÜNING F, et al.ε-Fe3N:magnetic structure, magnetization and temperature dependent disorder of nitrogen[J].J Alloy Compd, 1999, 288(1/2):79-87. http://d.old.wanfangdata.com.cn/Periodical/gncl201004014 [10] PANDA R N, GAJBHIYE N S.Magnetic properties of nanocrystalline γ'-Fe4N and ε-Fe3N synthesized by citrate route[J].IEEE T Magn, 1998, 34(2):542-548. doi: 10.1109/20.661488 [11] MILAD I K, SMITH K J, WONG P C, et al.A comparison of bulk metal nitride catalysts for pyridine hydrodenitrogenation[J].Catal Lett, 1998, 52(1):113-119. doi: 10.1023/A%3A1019071420213 [12] GUO K, RAU D, TOFFOLETTI L, et al.Ternary metastable nitrides ε-Fe2TMN (TM=Co, Ni):high-pressure, high-temperature synthesis, crystal structure, thermal stability, and magnetic properties[J].Chem Mater, 2012, 24(23):4600-4606. doi: 10.1021/cm3031297 [13] LEI L, YIN W, JIANG X, et al.Synthetic route to metal nitrides:high-pressure solid-state metathesis reaction[J].Inorg Chem, 2013, 52(23):13356-13362. doi: 10.1021/ic4014834 [14] YIN W, LEI L, JIANG X, et al.High pressure synthesis and properties studies on spherical bulk ε-Fe3N[J].High Pressure Res, 2014, 34(3):317-326. doi: 10.1080/08957959.2014.944910 [15] KOJIMA Y, OHFUJI H.Structure and stability of carbon nitride under high pressure and high temperature up to 125GPa and 3 000K[J].Diam Relat Mater, 2013, 39:1-7. doi: 10.1016/j.diamond.2013.07.006 [16] OHFUJI H, YAMAMOTO M.EDS quantification of light elements using osmium surface coating[J].J Miner Petrol Sci, 2015, 110(4):189-195. doi: 10.2465/jmps.141126 [17] HOHENBERG P, KOHN W.Inhomogeneous electron gas[J].Phys Rev B, 1964, 136(3):864-871. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0225733276/ [18] KOHN W, SHAM L J.Quantum density oscillations in an inhomogeneous electron gas[J].Phys Rev A, 1965, 137(3):1697-1705. doi: 10.1103-PhysRev.137.A1697/ [19] PERDEW J P, BURKE K, ERNZERHOF M.Generalized gradient approximation made simple[J].Phys Rev Lett, 1996, 77(18):3865-3868. doi: 10.1103/PhysRevLett.77.3865 [20] GIANNOZZI P, BARONI S, BONINI N, et al.Quantum espresso:a modular and open-source software project for quantum simulations of materials[J].J Phys Condens Mat, 2009, 21(39):395-502. doi: 10.1088-0953-8984-21-39-395502/ [21] GOTOU H, YAGI T, LIZUKA R, et al.Application of X-ray radiography to study the segregation process of iron from silicate under high pressure and high temperature[J].High Pressure Res, 2015, 35(2):130-138. doi: 10.1080/08957959.2015.1028932 -







 下载:
下载: