First-Principles Molecular Dynamics Study of the Structure of MgSiO3 Melt at High Temperatures and High Pressures
-
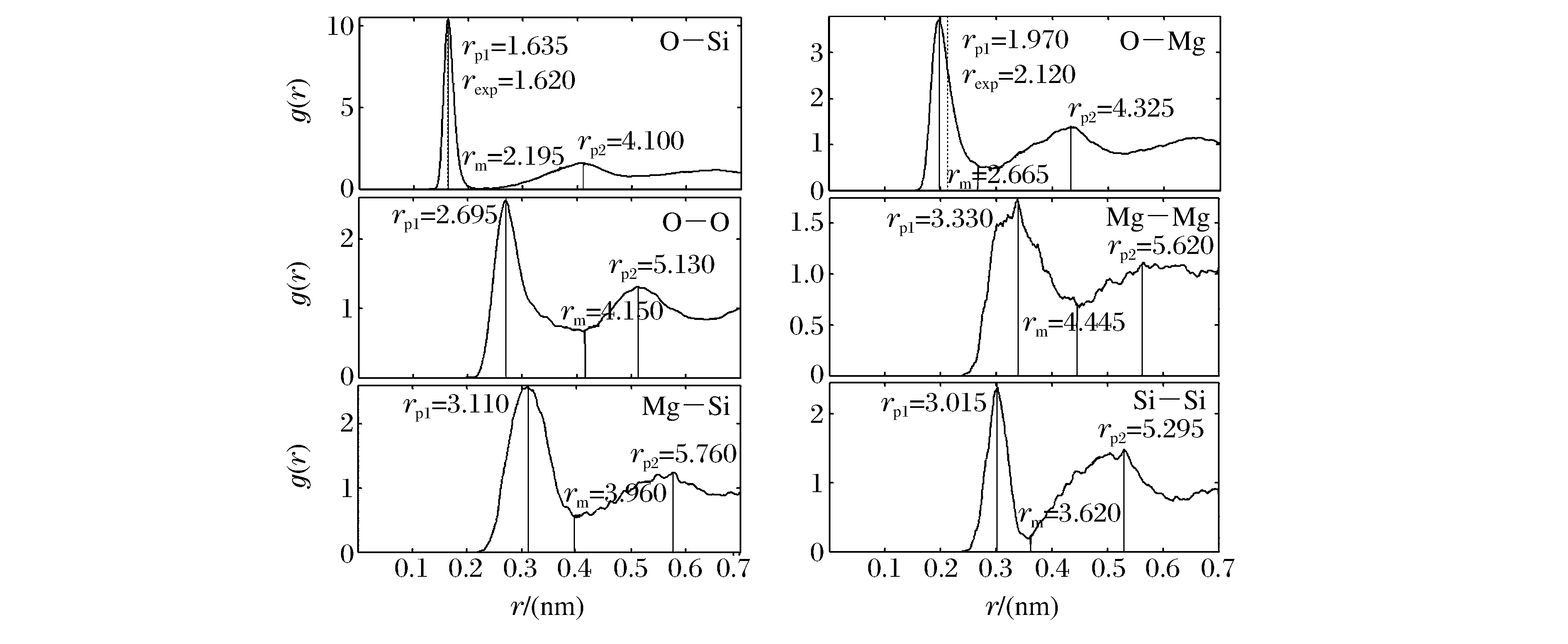
摘要: 基于第一性原理分子动力学方法,计算了MgSiO3熔体在0~144 GPa、2 000~6 000 K的微观结构及其随压力、温度的变化特征。计算的近零压2 000 K下O—Si、O—Mg和O—O对分布函数的第一峰值位置分别为0.163 5、0.197 0和0.269 5 nm,与实验结果吻合很好。随着压力和温度的变化,MgSiO3熔体结构发生了显著变化,尤其是随着压力增加,结构变得更致密;当密度为4.59 g/cm3时,原子间的平均键长随温度(小于5 000 K)增加而减小,在常压和更高的压力下,原子间的平均键长随温度变化不明显。在133 GPa、4 000 K条件下,MgSiO3熔体的O—Si、O—Mg和O—O平均键长分别为0.161 0、0.183 5和0.230 0 nm;从地表常压到核幔边界压力,平均Si—O配位数从4变到6,桥氧数目比例由31.3%增高到72.9%。MgSiO3熔体微观结构的认识对了解地幔内硅酸盐流体性质及其对地幔动力学的影响有重要意义。
-
关键词:
- MgSiO3熔体 /
- 第一性原理分子动力学 /
- 对分布函数 /
- 平均配位数
Abstract: The microstructures of MgSiO3 melt and their variation with temperature and pressure were investigated based on first-principles molecular dynamic simulations at high pressures (0-144 GPa) and high temperatures (2 000-6 000 K).The calculated first peak positions of the pair correlation function of O—Si, O—Mg and O—O under the condition of 0 GPa and 2 000 K are 0.163 5, 0.1 970 and 0.269 5 nm, respectively, which are consistent with the previous experimental values.As the pressure and temperature change, the structure of MgSiO3 melt undergoes a significant change.Especially when the pressure increases, the structure becomes denser.When the temperature is below 5 000 K, the average bond lengths between two atoms decrease with the increasing temperature with density 4.59 g/cm3.While under nomal or higher pressure, the average bond length change with the increasing temperature is not obvious.At 133 GPa and 4 000 K, the average bond lengths of O—Si, O—Mg and O—O are 0.161 0, 0.183 5 and 0.230 0 nm, respectively; the average Si—O coordination number increases from 4 to 6, and the number of bridging oxygen ratio increases from 31.3% to 72.9%, from atmospheric pressure to the core-mantle boundary.The knowledge of MgSiO3 melt microstructure is important to understand the mantle silicate fluid nature of mantle dynamics. -
表 1 MgSiO3熔体在不同温压下的PCF峰值位置
Table 1. The calculation positions of PCF peaks of MgSiO3 melt under different pressures and temperatures
(nm) ρ/(g/cm3) T/(K) O—O O—Mg O—Si rp1 rm rp2 rp1 rm rp2 rp1 rm rp2 2.71
This work2 000 0.269 5 0.415 0 0.513 0 0.197 0 0.266 5 0.432 5 0.163 5 0.219 5 0.413 5 3 000 0.272 0 0.392 5 0.505 0 0.197 0 0.275 0 0.436 5 0.162 5 0.236 0 0.411 5 4 000 0.267 0 0.397 5 0.513 5 0.196 0 0.302 0 0.413 5 0.165 0 0.241 0 0.403 5 5 000 0.275 0 0.388 0 0.508 5 0.191 5 0.292 0 0.420 0 0.164 0 0.255 0 0.402 5 6 000 0.272 0 0.388 0 0.528 0 0.203 5 0.294 5 0.419 5 0.164 0 0.226 5 0.404 5 5.16
This work3 000 0.229 0 0.303 5 0.436 5 0.184 0 0.279 0 0.392 5 0.162 0 0.228 0 0.383 0 4 000 0.230 0 0.302 0 0.436 0 0.183 5 0.269 0 0.388 5 0.161 0 0.231 5 0.371 5 6 000 0.224 5 0.308 0 0.438 5 0.185 0 0.272 5 0.384 5 0.159 0 0.233 0 0.367 5 Waseda[10] 1 970 0.212 0 0.162 0 2.57
Karki[5]2 500 0.267 5 0.397 5 0.505 5 0.196 5 0.290 5 0.429 5 0.162 5 0.236 5 0.389 0 3 000 0.267 5 0.397 5 0.507 0 0.196 5 0.292 0 0.429 5 0.162 5 0.237 5 0.383 5 4 000 0.267 5 0.388 5 0.507 5 0.196 5 0.295 0 0.430 5 0.162 5 0.243 5 0.386 0 5 000 0.267 5 0.392 5 0.510 5 0.196 5 0.307 5 0.432 5 0.162 5 0.246 5 0.384 0 6 000 0.266 5 0.389 5 0.515 0 0.195 5 0.307 5 0.434 5 0.162 5 0.247 5 0.371 0 5.14
Karki[5]3 000 0.231 5 0.301 5 0.427 5 0.184 5 0.262 5 0.400 5 0.162 5 0.234 5 0.375 5 4 000 0.229 5 0.301 0 0.430 5 0.184 5 0.272 5 0.395 5 0.162 0 0.229 5 0.375 0 6 000 0.225 5 0.306 0 0.432 5 0.183 5 0.275 0 0.389 5 0.159 5 0.237 5 0.370 5 -
[1] 莫宣学.岩浆熔体结构[J].地质科技情报, 1985, 4: 21-23. http://www.cnki.com.cn/Article/CJFDTotal-DZKQ198502005.htmMo X X. Structure of magmatic melt[J]. Geological Science and Technology Information, 1985, 4: 21-23. (in Chinese) http://www.cnki.com.cn/Article/CJFDTotal-DZKQ198502005.htm [2] 朱承峰, 张传清.硅酸盐熔体结构学[M].北京: 地质出版社, 1996.Zhu C F, Zhang C Q. Structure of Silicate Melts[M]. Beijing: Geological Press, 1996. (in Chinese) [3] Mysen B O. The structure of silicate melts[J]. Ann Rev Earth Planet Sci, 1983, 11: 75-97. doi: 10.1146/annurev.ea.11.050183.000451 [4] Mysen B O. Relationships between silicate melt structure and petrologic processes[J]. Earth-Sci Rev, 1990, 27: 281-365. doi: 10.1016/0012-8252(90)90055-Z [5] Karki B B. First-principles molecular dynamics simulations of silicate melts: Structural and dynamical properties[J]. Rev Mineral Geochem, 2010, 71(1): 355-389. http://www.researchgate.net/publication/250130814_First-Principles_Molecular_Dynamics_Simulations_of_Silicate_Melts_Structural_and_Dynamical_Properties [6] 龚自正, 谢鸿森, 费英伟, 等.下地幔矿物研究及其进展[J].地学前缘, 2005, 12(1): 3-22. http://www.cnki.com.cn/Article/CJFDTotal-DXQY200501000.htmGong Z Z, Xie H S, Fei Y W, et al. A review of recent advances on the Earth's lower mantle[J]. Earth Science Frontiers, 2005, 12(1): 3-22. (in Chinese) http://www.cnki.com.cn/Article/CJFDTotal-DXQY200501000.htm [7] Levitas V I, Ma Y, Selvi E, et al. High-density amorphous phase of silicon carbide obtained under large plastic shear and high pressure[J]. Phys Rev B, 2012, 85: 054114-5. doi: 10.1103/PhysRevB.85.054114 [8] Stixrude L, Karki B B. Structure and freezing of MgSiO3 liquid in Earth's lower mantle[J]. Science, 2005, 310: 297-299. doi: 10.1126/science.1116952 [9] 孙樯, 郑海飞, 谢鸿森, 等.硅酸盐熔体结构研究进展及意义[J].岩石学报, 2001, 17(2): 332-336. http://www.cnki.com.cn/Article/CJFDTotal-YSXB200102018.htmSun Q, Zheng H F, Xie H S, et al. The advance in silicate melt structure research and its significance[J]. Acta Petrologica Sinica, 2001, 17(2): 332-336. (in Chinese) http://www.cnki.com.cn/Article/CJFDTotal-YSXB200102018.htm [10] Waseda Y, Toguri J M. Structure of silicate melts determined by X-ray diffraction[C]//Marumo F. Dynamics Process of Material Transport and Transformation in Earth's Interior. Tokyo: Terra Sci, 1990: 37-51. [11] Leea S K, Linb J F, Caid Y Q, et al. X-ray Raman scattering study of MgSiO3 glass at high pressure: Implication for triclustered MgSiO3 liquid in Earth's mantle[J]. PNAS, 2008, 105(23): 7925-7929. doi: 10.1073/pnas.0802667105 [12] Yamada A, Inoue T, Urakawa S, et al. In situ X-ray experiment on the structure of hydrous Mg-silicate liquid under high pressure and high temperature[J]. Geophys Res Lett, 2007, 34(10): L10303. doi: 10.1029/2006GL028823 [13] George A M, Stebbins J F. Structure and dynamics of magnesium in silicate melts: A high-temperature 25Mg NMR study[J]. Am Mineral, 1998, 83: 1022-1029. doi: 10.2138/am-1998-9-1010 [14] Nobumasa F, Shino Y, Takehiko Y. Exploratory studies of silicate melt structure at high pressures and temperatures by in situ X-ray diffraction[J]. J Geophys Res, 2004, 109: B03203. doi: 10.1029/2003JB002650/full [15] Wilding M C, Benmore C J, Weber J K R. In situ diffraction studies of magnesium silicate liquids[J]. J Mater Sci, 2008, 43: 4707-4713. doi: 10.1007/s10853-007-2356-5 [16] Kubicki J D, Lasaga A C. Molecular dynamics simulations of pressure and temperature effects on MgSiO3 and Mg2SiO4 melts and glasses[J]. Phys Chem Miner, 1991, 17: 661-673. doi: 10.1007/BF00202236 [17] Martin G B, Spera F J, Ghiorso M S, et al. Structure, thermodynamic and transport properties of molten Mg2SiO4: Molecular dynamics simulations and model EOS[J]. Am Mineral, 2009, 94: 693-703. doi: 10.2138/am.2009.3087 [18] Stixrude L, Koker N D, Sun N, et al. Thermodynamics of silicate liquids in deep Earth[J]. Earth Planet Sci Lett, 2009, 278: 226-232. doi: 10.1016/j.epsl.2008.12.006 [19] Karki B B, Bhattarai D, Mookherjee M, et al. Visualization-based analysis of structural and dynamical properties of simulated hydrous silicate melt[J]. Phys Chem Miner, 2010, 37: 103-117. doi: 10.1007/s00269-009-0315-1 [20] Mookherjee M, Stixrude L, Karki B B. Hydrous silicate melt at high pressure[J]. Nature, 2008, 452: 983-986. doi: 10.1038/nature06918 [21] Karki B B, Stixrude L. Viscosity of MgSiO3 liquid at Earth's mantle conditions: Implications for an early magma ocean[J]. Science, 2010, 328: 740-742. doi: 10.1126/science.1188327 [22] Nevins D, Spera F J, Ghiorso M S. Shear viscosity and diffusion in liquid MgSiO3: Transport properties and implications for terrestrial planet magma ocean[J]. Am Mineral, 2009, 94: 975-980. doi: 10.2138/am.2009.3092 [23] Lacks D J, Rear D B, Orman J A V. Molecular dynamics investigation of viscosity, chemical diffusivities and partial molar volumes of liquids along the MgO-SiO2 joins as functions of pressure[J]. Geochim Cosmochim Ac, 2007, 71: 1312-1323. doi: 10.1016/j.gca.2006.11.030 [24] Wan J T K, Duffy T S, Sandro S, et al. First principles study of density, viscosity, and diffusion coefficients of liquid MgSiO3 at conditions of the Earth's deep mantle[J]. J Geophys Res-Solid Earth, 2007, 112: B03208. doi: 10.1029/2005JB004135/full [25] Kresse G, Furthmuller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Comp Mater Sci, 1996, 6: 15-50. doi: 10.1016/0927-0256(96)00008-0 [26] Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Phys Rev B, 1999, 59: 1758-1775. http://pubs.acs.org/servlet/linkout?suffix=ref44/cit44b&dbid=16&doi=10.1021%2Facs.langmuir.5b02784&key=10.1103%2FPhysRevB.59.1758 [27] Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Phys Rev Lett, 1996, 77: 3865-3868. doi: 10.1103/PhysRevLett.77.3865 [28] Nose S. A unified formulation of the constant temperature molecular dynamics[J]. J Chem Phys, 1984, 81: 511-519. doi: 10.1063/1.447334 [29] Williams Q, Garnero E J. Seismic evidence for partial melt at the base of Earth's mantle[J]. Science, 1996, 273: 1528-1530. doi: 10.1126/science.273.5281.1528 [30] Lange R A, Carmichael I S E. Densities of Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-TiO2-SiO2 liquids: New measurements and derived partial molar properties[J]. Geochim Cosmochim Acta, 1987, 51: 2931-2946. doi: 10.1016/0016-7037(87)90368-1 [31] Sanchez-Valle C, Bass J D. Elasticity and pressure induced structural changes in vitreous MgSiO3-enstatite to lower mantle pressures[J]. Earth Planet Sci Lett, 2010, 295: 523-530. doi: 10.1016/j.epsl.2010.04.034 [32] Akins J A, Luo S N, Asimow P D, et al. Shock-induced melting of MgSiO3 perovskite and implications for melts in Earth's lowermost mantle[J]. Geophys Res Lett, 2004, 31: L14612. doi: 10.1029/2004GL020237 [33] Zeng Q S, Sheng H W, Ding Y, et al. Long-range topological order in metallic glass[J]. Science, 2011, 332: 1404-1406. doi: 10.1126/science.1200324 [34] Tian H, Liu H, Zhang C, et al. Ab initio molecular dynamics simulation of binary Ni62.5Nb37.5 bulk metallic glass: Validation of the cluster-plus-glue-atom model[J]. J Mater Sci, 2012, 47: 7628-7634. doi: 10.1007/s10853-012-6306-5 [35] Spera F J, Ghiorso M S, Nevins D. Structure, thermodynamic and transport properties of liquid MgSiO3: Comparison of molecular models and laboratory results[J]. Geochim Cosmochim Acta, 2011, 75: 1272-1296. doi: 10.1016/j.gca.2010.12.004 -







 下载:
下载: