First-Principles Study on Mechanical Properties of Sc, Ti, V, Zr-Doped Cr2B3 at High Pressure
-
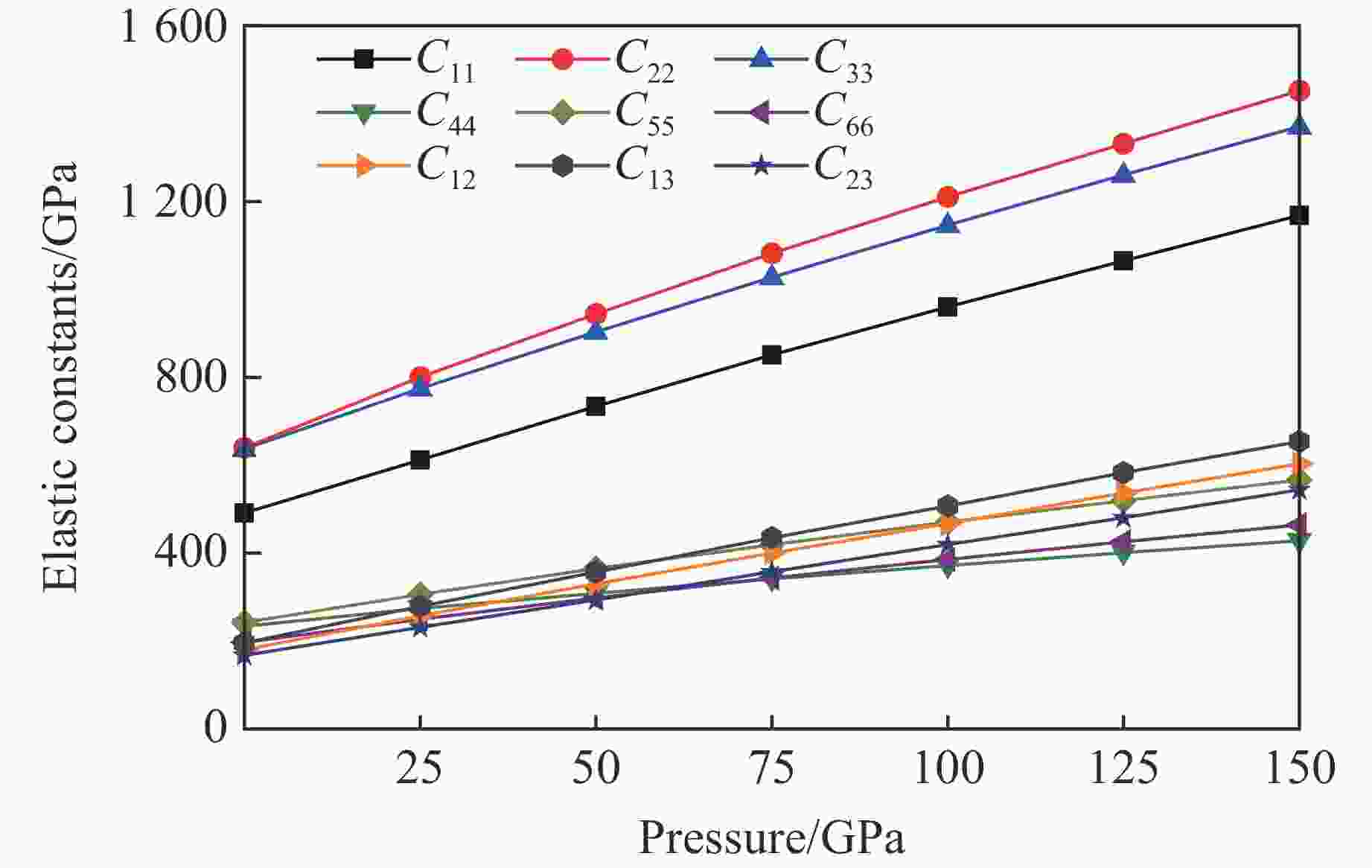
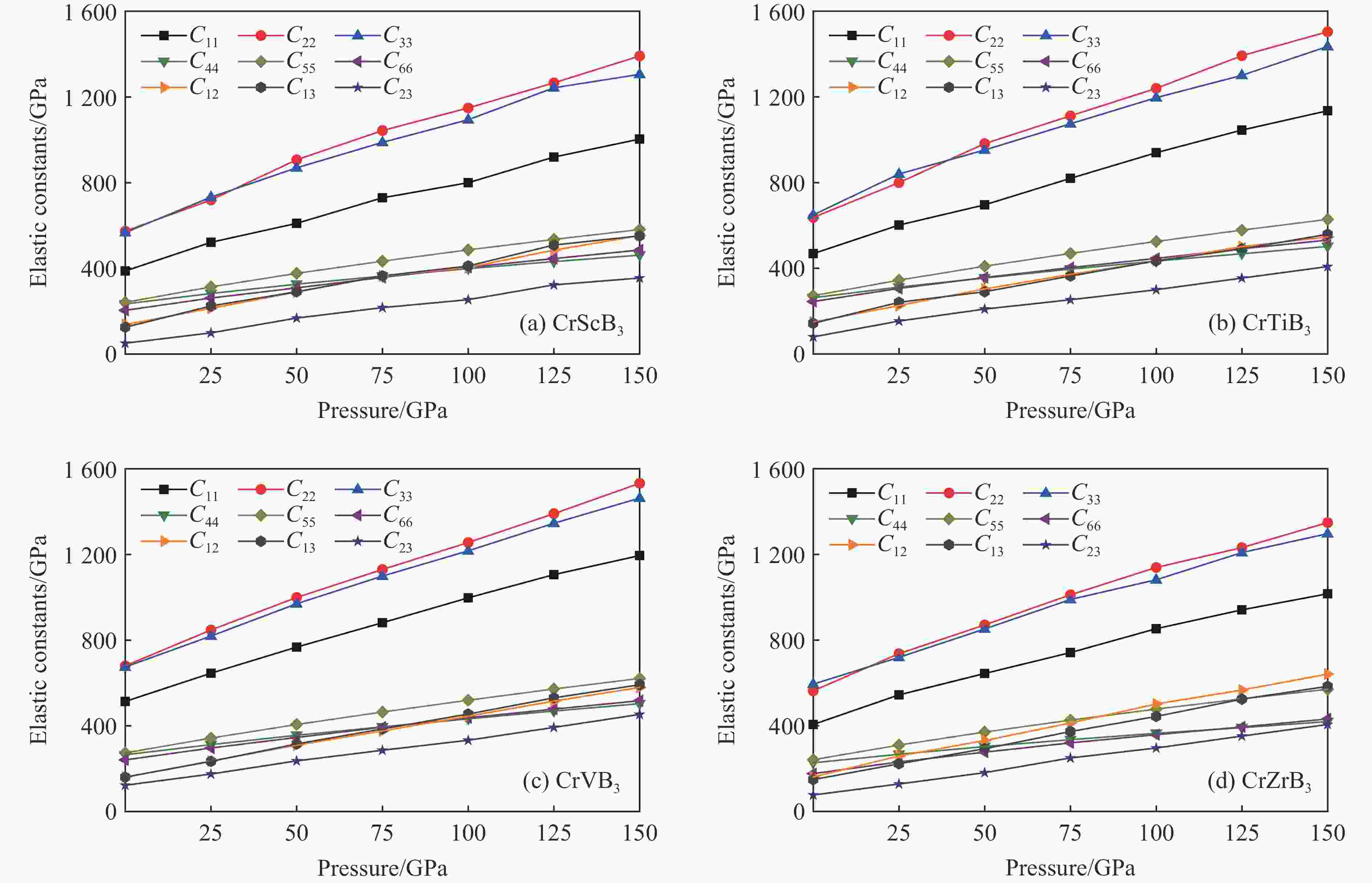
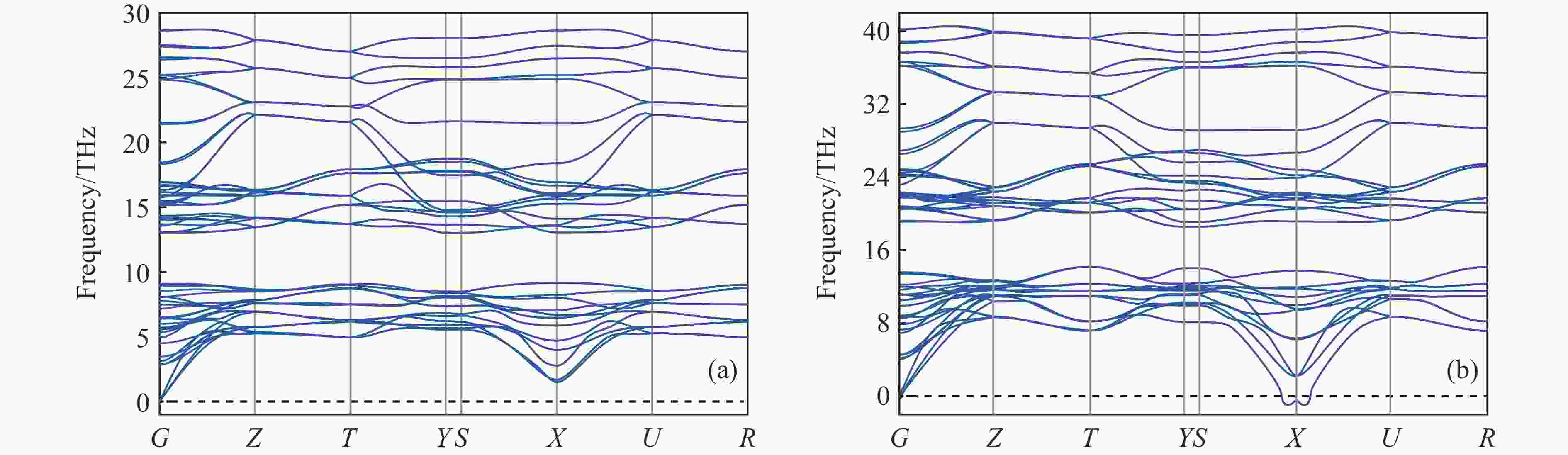
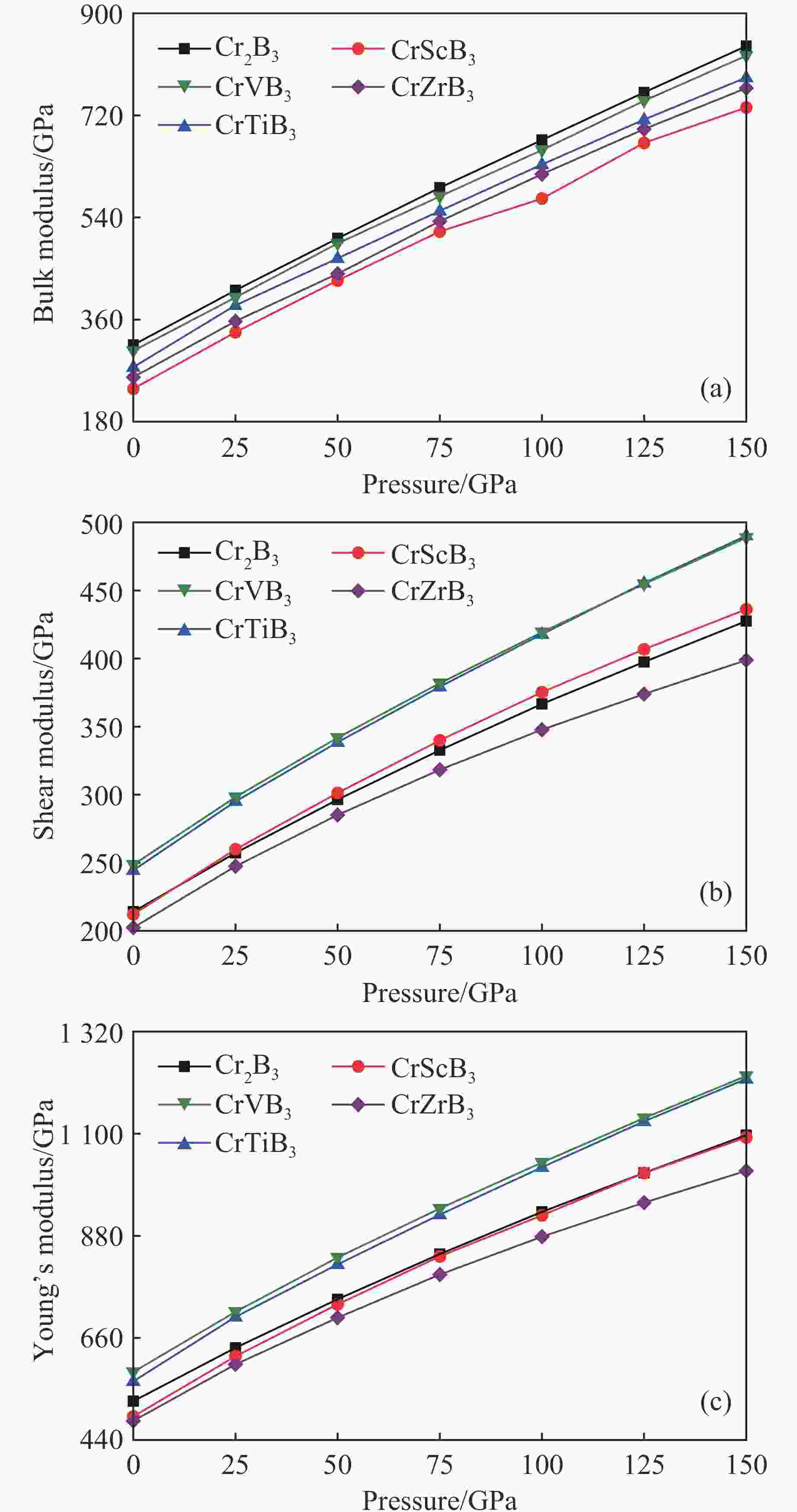
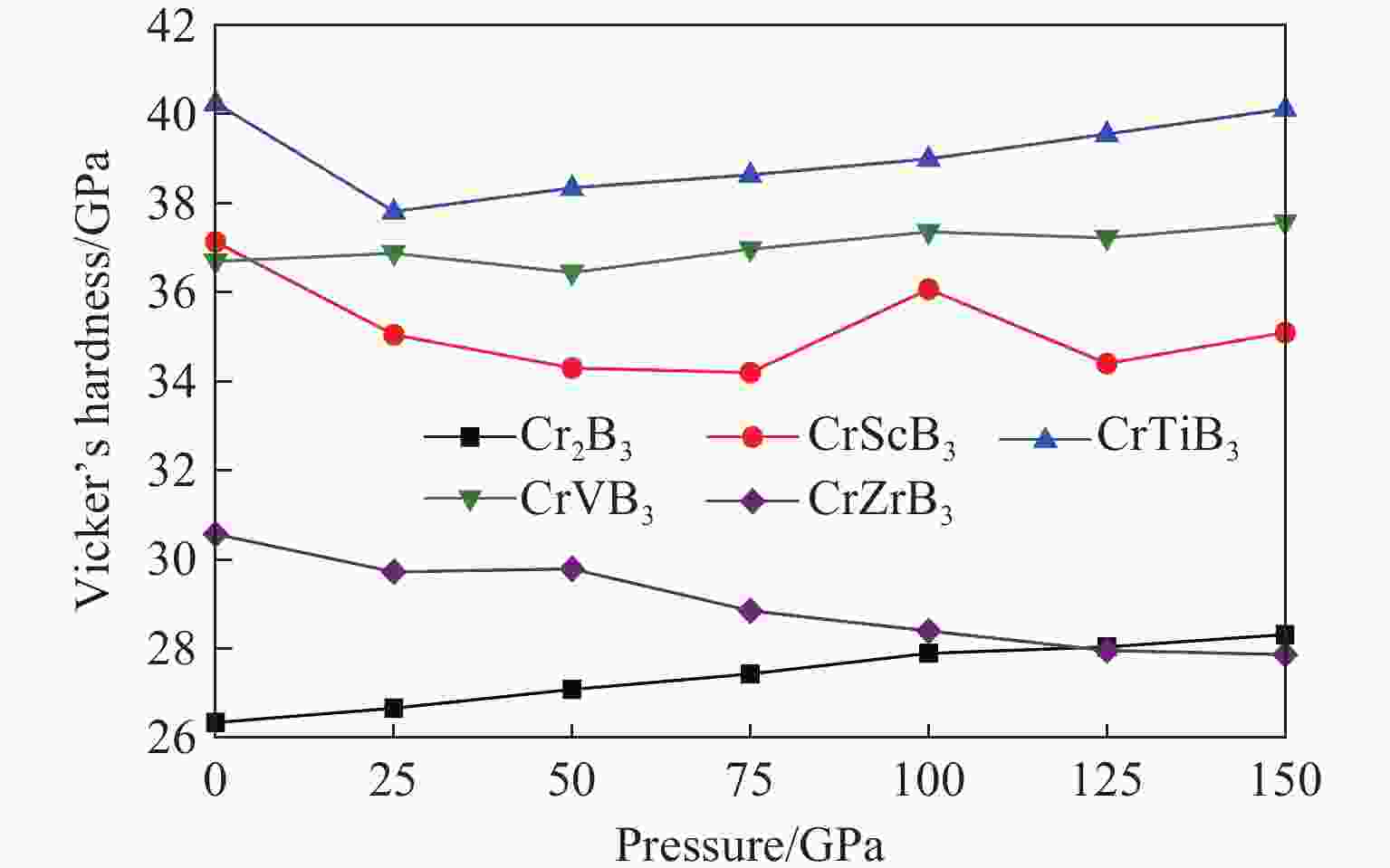
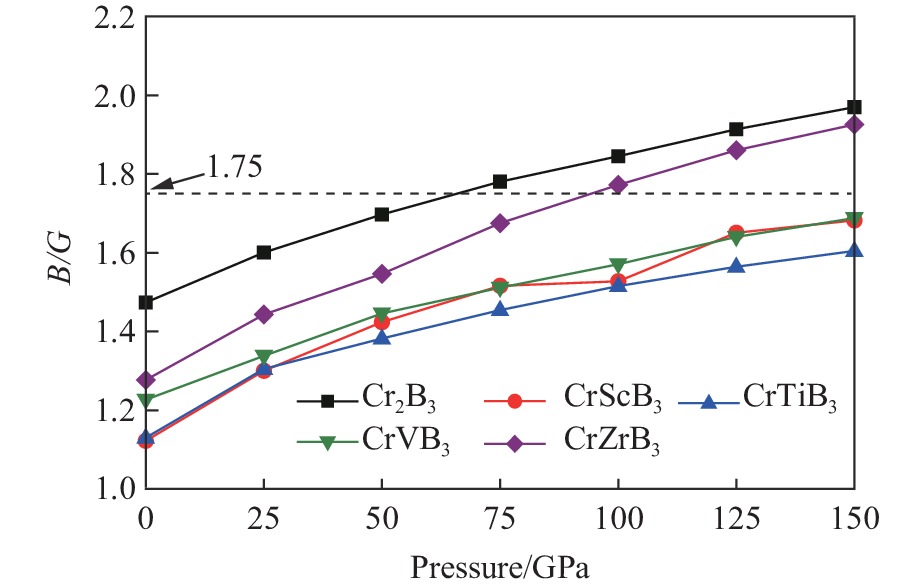
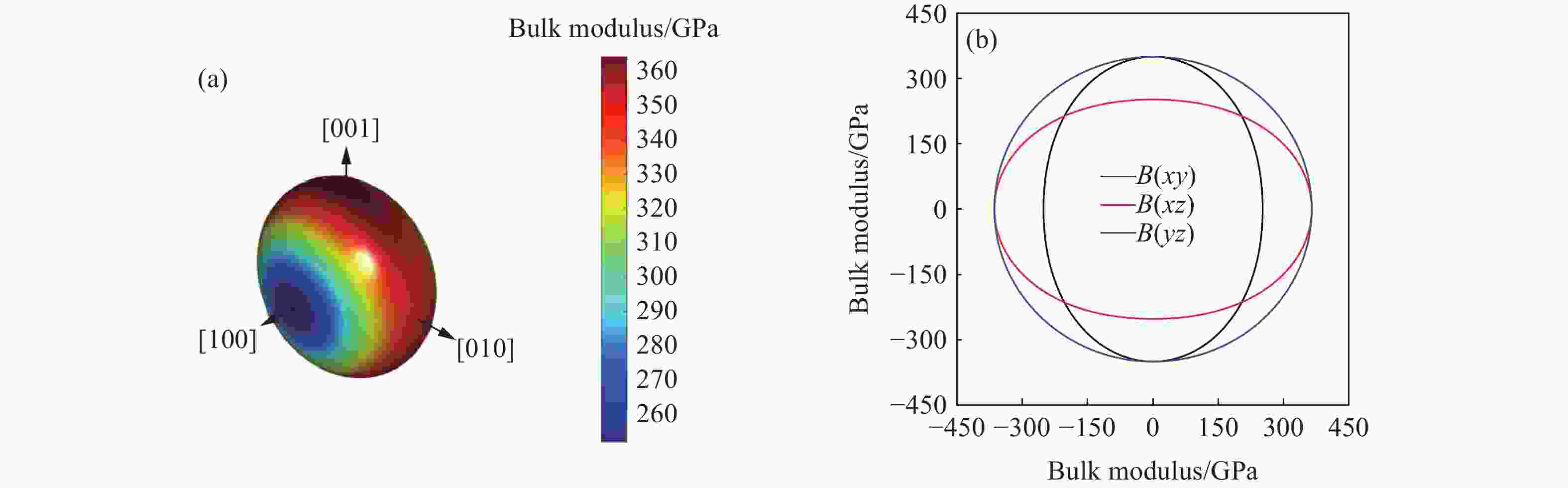
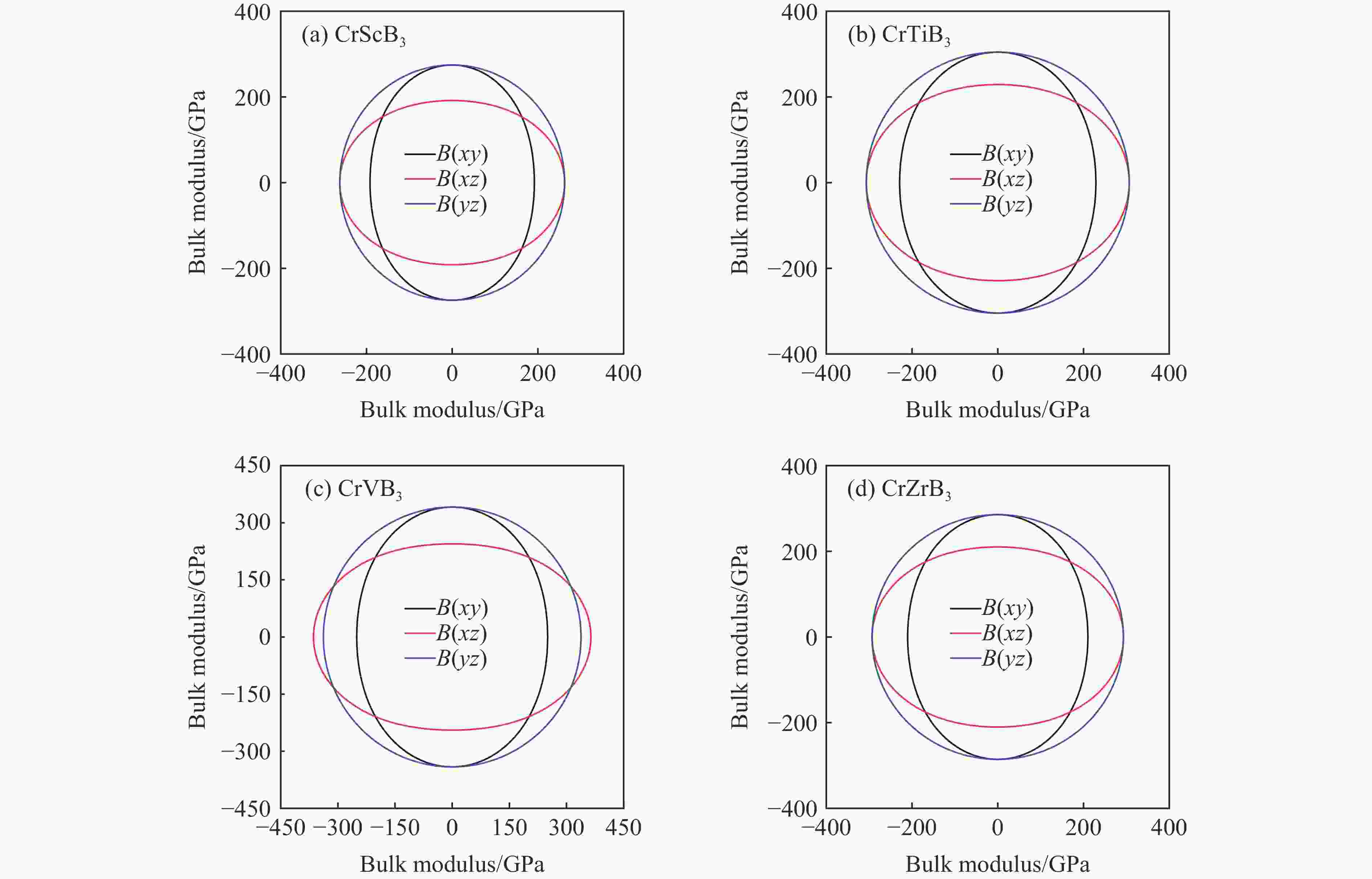
摘要: 采用基于密度泛函理论的第一性原理方法,计算了掺杂Sc、Ti、V和Zr的Cr2B3在零压下的晶体结构和电子结构及其在0~150 GPa压力范围内的弹性常数和维氏硬度。结果表明:Cr2B3及其掺杂化合物均具备力学稳定性;在零压下,添加Sc、Ti、V和Zr元素均可提高Cr2B3的维氏硬度,其中Ti掺杂Cr2B3的硬度由26.3 GPa提高至40.2 GPa,提高52.9%,达到超硬材料标准,且 Ti和V掺杂Cr2B3的剪切模量分别提高14.3%和16.2%,杨氏模量分别提高8.2%和12.0%;由电子结构分析可知,Sc、Ti、V和 Zr元素可以加强B与B之间的电子局域化程度,从而增强共价键结合强度,使Cr2B3的硬度升高;Cr2B3的弹性常数、体积弹性模量、剪切模量、杨氏模量以及硬度随着压力的增加而增加,但其硬度仍较低,150 GPa下仅为28.3 GPa,而掺杂V的Cr2B3的硬度在整个压力范围内约为37 GPa。研究结果可为Cr2B3在高压等特殊条件下的应用提供理论参考。Abstract: By using first-principles method based on density functional theory, the crystal structures and the electronic structures of Sc, Ti, V and Zr element doped Cr2B3 under zero pressure, and their elastic constants and hardness in the pressure range of 0−150 GPa are calculated. According to the calculation, Cr2B3 and its doped compounds all meet the mechanical stability. At zero pressure, the Vicker’s hardness of Cr2B3 can be improved by adding Sc, Ti, V and Zr elements, in which the hardness of Ti-doped Cr2B3 is increased from 26.3 GPa to 40.2 GPa, increasing by 52.9%, reaching the standard of superhard materials, and the shear moduli of Ti and V doped Cr2B3 increase by 14.3% and 16.2%, respectively, and the Young’s moduli of the Ti and V doped Cr2B3 increase by 8.2% and 12.0%, respectively. The analysis of electronic structures shows that Sc, Ti, V and Zr can enhance the degree of electronic localization between B and B, thus enhance the covalent bonding strength and increase the hardness of Cr2B3. In addition, the elastic constants, the bulk elastic modulus, the shear modulus, the Young’s modulus and the hardness of Cr2B3 increase with pressure augmentation, but even so, the hardness is still low, merely 28.3 GPa at 150 GPa, while the hardness of the V element doped Cr2B3 is nearly constant (about 37 GPa) in the pressure range of 0−150 GPa. This work provides a theoretical reference for an extending application of Cr2B3 in special conditions, such as high pressure.
-
Key words:
- Cr2B3 /
- doping /
- elastic constants /
- hardness /
- high pressure
-
图 15 零压下(a) Cr2B3、(b) CrScB3、(c) CrTiB3、(d) CrVB3、(e) CrZrB3在(100)平面的 ELF 以及零压下(f) Cr2B3、(g) CrScB3、(h) CrTiB3、(i) CrVB3和(j) CrZrB3在(
$00 \overline 1 $ )平面的 ELFFigure 15. Electronic local functions contours for (a) Cr2B3, (b) CrScB3, (c) CrTiB3, (d) CrVB3 and (e) CrZrB3 in plane (100) at 0 GPa, and electronic local functions contours for (f) Cr2B3, (g) CrScB3, (h) CrTiB3, (i) CrVB3 and (j) CrZrB3 in plane (
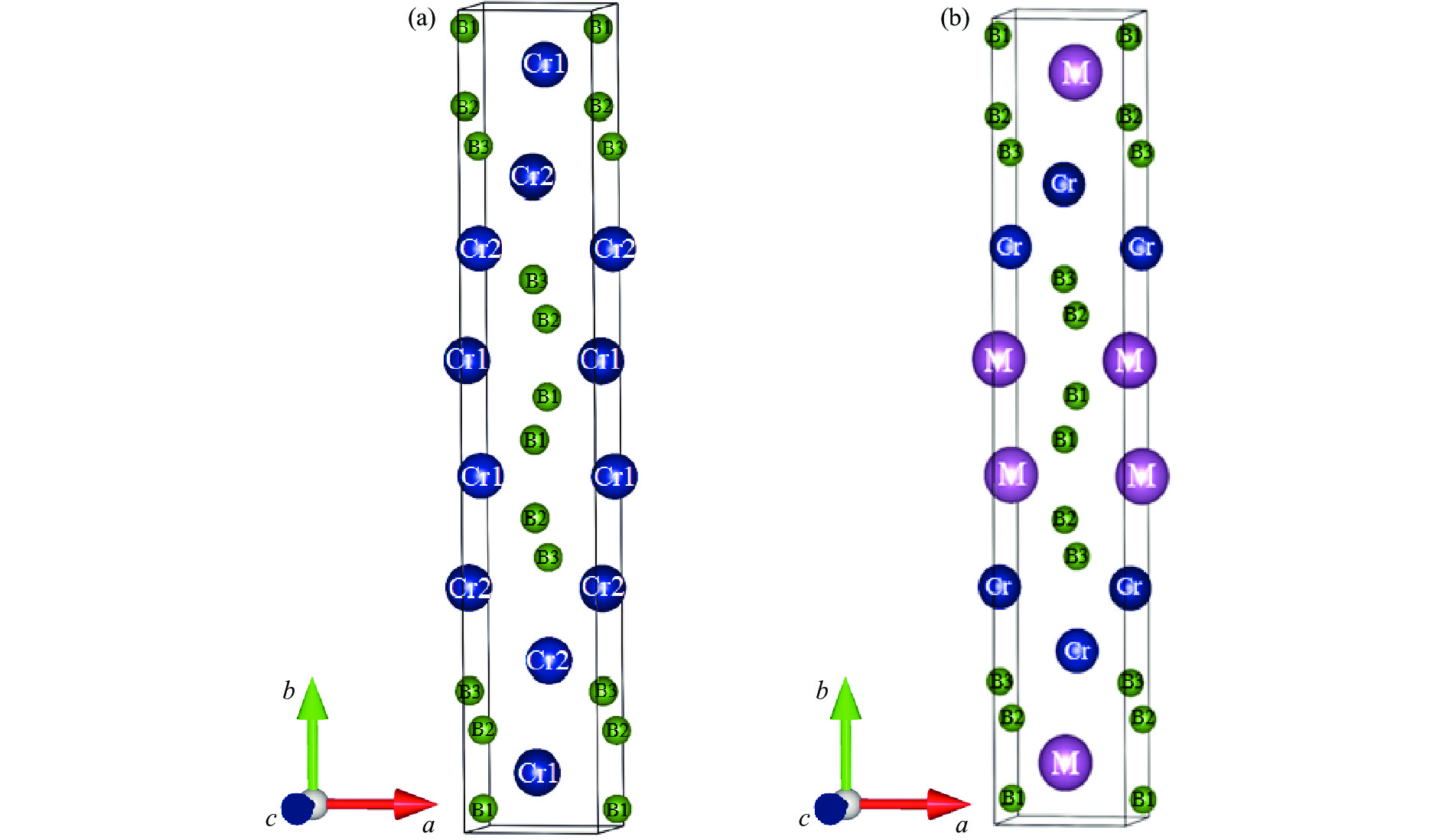
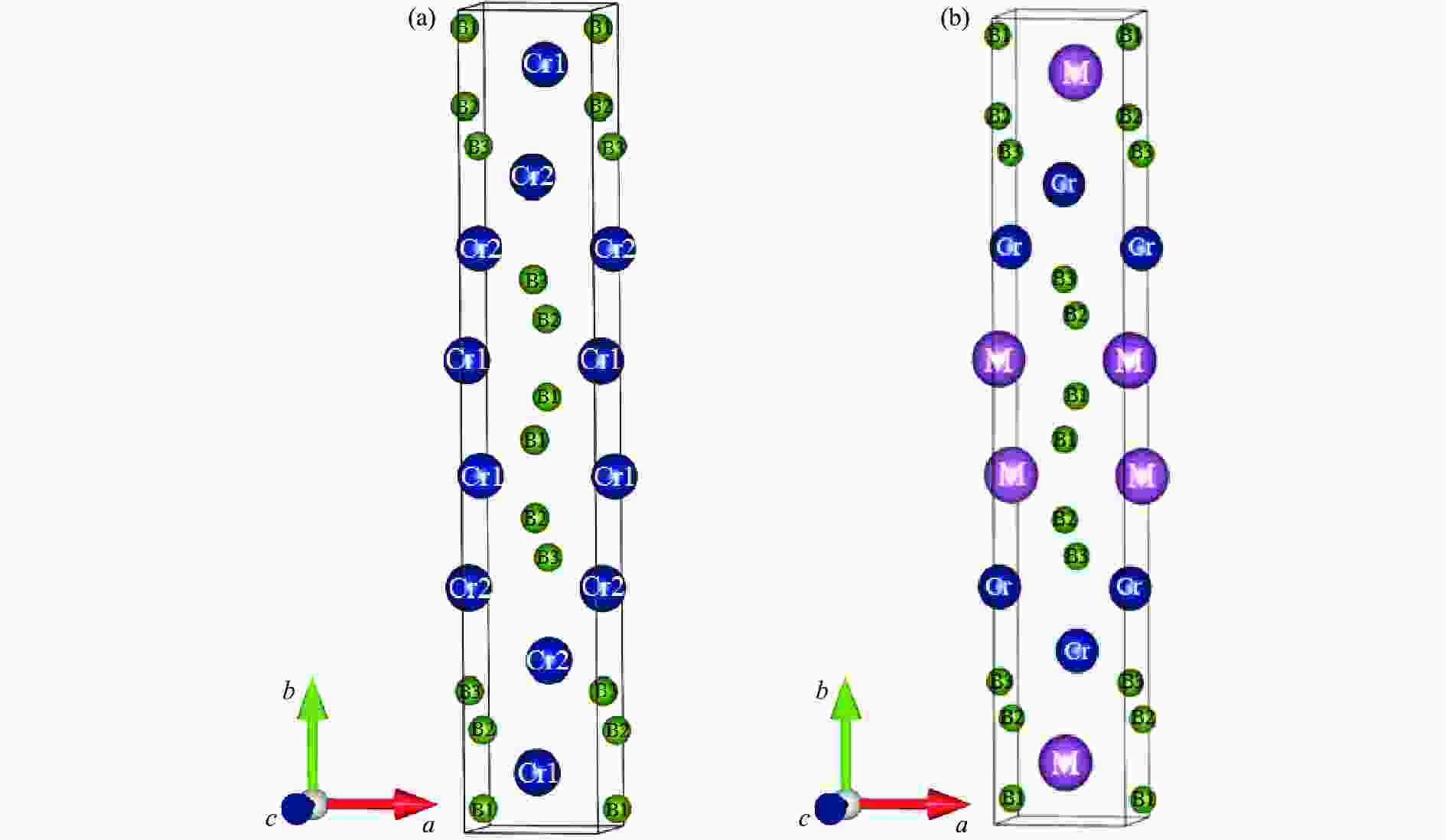
$00 \overline 1 $ ) at 0 GPa表 1 零压下Cr2B3及掺杂结构CrMB3(M=Sc, Ti, V, Zr)的晶格常数、形成焓及掺杂结构的形成能
Table 1. Lattice constants, formation enthalpy of Cr2B3 and CrMB3 (M = Sc, Ti, V, Zr), and impurity formation energy ofCrMB3 (M=Sc, Ti, V, Zr) at zero pressure
Compounds Doping-site position Space group Lattice constants Ef/eV ΔH/(eV∙atom−1) a/Å b/Å c/Å Cr2B3 Cmcm 2.8983 18.0464 2.9286 −0.4731 CrScB3 Cr1 Cmcm 3.2207 18.5222 3.0293 −0.9713 −0.6653 Cr2 CrTiB3 Cr1 Cmcm 3.0648 18.1669 2.9746 −1.9192 −0.8561 Cr2 Cmcm 3.0541 18.5900 2.9883 −0.6876 −0.6118 CrVB3 Cr1 Cmcm 2.9546 18.0874 2.9436 −1.1856 −0.7092 Cr2 Cmcm 2.9547 18.2254 2.9489 −0.6797 −0.6101 CrZrB3 Cr1 Cmcm 3.2972 18.5872 3.0702 −1.2236 −0.7181 Cr2 Cmcm 3.1763 20.0136 3.0524 0.1705 −0.4424 表 2 Cr2B3中B―B键的键长以及布居数随压力的变化
Table 2. Pressure dependence of B―B bond length and population for Cr2B3
Pressure/GPa Bond length/Å Population B1―B1 B1―B2 B2―B3 B1―B1 B1―B2 B2―B3 0 1.69586 1.71971 1.75978 1.59 0.63 1.36 25 1.66181 1.68355 1.72369 1.62 0.64 1.40 50 1.63474 1.65559 1.69462 1.65 0.65 1.44 75 1.61237 1.63214 1.67012 1.68 0.66 1.47 100 1.59298 1.61225 1.64931 1.70 0.67 1.50 125 1.57589 1.59506 1.63098 1.73 0.68 1.53 150 1.56058 1.57950 1.61442 1.75 0.69 1.56 -
[1] CAO A H, ZHAO W J, ZHOU Q Y, et al. A superhard allotrope of carbon: ibam-C and its BN phase [J]. Chemical Physics Letters, 2019, 714: 119–124. doi: 10.1016/j.cplett.2018.10.079 [2] FENG S Q, YANG Y, GUO F, et al. Structural, elastic, electronic and hardness properties of osmium diboride predicted from first principles calculations [J]. Journal of Alloys and Compounds, 2020, 844: 156098. doi: 10.1016/j.jallcom.2020.156098 [3] WANG C C, TAO Q, DONG S S, et al. Synthesis and mechanical character of hexagonal phase δ-WN [J]. Inorganic Chemistry, 2017, 56(7): 3970–3975. doi: 10.1021/acs.inorgchem.6b03041 [4] CAI Y X, XIONG J M, LIU Y B, et al. Electronic structure and chemical hydrogen storage of a porous sp3 tetragonal BC2N compound [J]. Journal of Alloys and Compounds, 2017, 724: 229–233. doi: 10.1016/j.jallcom.2017.06.343 [5] MOHAMMADI R, LECH A T, XIE M, et al. Tungsten tetraboride, an inexpensive superhard material [J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(27): 10958–10962. doi: 10.1073/pnas.1102636108 [6] KANER R B, GILMAN J J, TOLBERT S H. Designing superhard materials [J]. Science, 2005, 308(5726): 1268–1269. doi: 10.1126/science.1109830 [7] GOU H Y, LI Z P, NIU H, et al. Unusual rigidity and ideal strength of CrB4 and MnB4 [J]. Applied Physics Letters, 2012, 100(11): 111907. doi: 10.1063/1.3692777 [8] CHONG X Y, JIANG Y H, ZHOU R, et al. Elastic properties and electronic structures of Cr xB y as superhard compounds [J]. Journal of Alloys and Compounds, 2014, 610: 684–694. doi: 10.1016/j.jallcom.2014.05.010 [9] ANDERSSON S, LUNDSTRÖM T. The crystal structure of CrB4 [J]. Acta Chemica Scandinavica, 1968, 22(10): 3103–3110. [10] KOTZOTT D, ADE M, HILLEBRECHT H. Synthesis and crystal structures of α- and β- modifications of Cr2IrB2 containing 4-membered B4 chain fragments, the τ-boride Cr7.9Ir14.1B6 and orthorhombic Cr2B [J]. Solid State Sciences, 2008, 10(3): 291–302. doi: 10.1016/j.solidstatesciences.2007.09.014 [11] OKADA S, ATODA T, HIGASHI I. Structural investigation of Cr2B3, Cr3B4, and CrB by single-crystal diffractometry [J]. Journal of Solid State Chemistry, 1987, 68(1): 61–67. doi: 10.1016/0022-4596(87)90285-4 [12] GIANOGLIO C, PRADELLI G, VALLINO M. Solid state equilibria in the Cr-Fe-B system at the temperature of 1 373 K [J]. Metallurgical Science and Tecnology, 1983, 1(2): 51–57. [13] WONG-NG W, MCMURDIE H F, PARETZKIN B, et al. Reference X-ray diffraction powder patterns of fifteen ceramic phases [J]. Powder Diffraction, 1987, 2(4): 257–265. doi: 10.1017/S0885715600012926 [14] NIU H Y, WANG J Q, CHEN X Q, et al. Structure, bonding, and possible superhardness of CrB4 [J]. Physical Review B, 2012, 85(14): 144116. doi: 10.1103/PhysRevB.85.144116 [15] ZHANG Y K, WU L L, WAN B, et al. Structural variety beyond appearance: high-pressure phases of CrB4 in comparison with FeB4 [J]. Physical Chemistry Chemical Physics, 2016, 18(4): 2361–2368. doi: 10.1039/C5CP06745F [16] WANG S, YU X, ZHANG J, et al. Crystal structures, elastic properties, and hardness of high-pressure synthesized CrB2 and CrB4 [J]. Journal of Superhard Materials, 2014, 36(4): 279–287. doi: 10.3103/S1063457614040066 [17] OKADA S, KUDOU K, IIZUMI K, et al. Single-crystal growth and properties of CrB, Cr3B4, Cr2B3 and CrB2 from high-temperature aluminum solutions [J]. Journal of Crystal Growth, 1996, 166(1/2/3/4): 429–435. [18] MIAO N H, SA B S, ZHOU J, et al. Theoretical investigation on the transition-metal borides with Ta3B4-type structure: a class of hard and refractory materials [J]. Computational Materials Science, 2011, 50(4): 1559–1566. doi: 10.1016/j.commatsci.2010.12.015 [19] XING W D, MENG F Y, YU R. Strengthening materials by changing the number of valence electrons [J]. Computational Materials Science, 2017, 129: 252–258. doi: 10.1016/j.commatsci.2016.12.037 [20] ZHANG Y M, LIU D, ZHAO Y H, et al. Physical properties and electronic structure of Cr2B under pressure [J]. Physica Status Solidi (B), 2021, 258(2): 2000212. doi: 10.1002/pssb.202000212 [21] DOVALE-FARELO V, TAVADZE P, VERSTRAETE M J, et al. Exploring the elastic and electronic properties of chromium molybdenum diboride alloys [J]. Journal of Alloys and Compounds, 2021, 866: 158885. doi: 10.1016/j.jallcom.2021.158885 [22] OKADA S, ATODA T, HIGASHI I, et al. Preparation of single crystals of a new boride Cr2B3 by the aluminium-flux technique and some of its properties [J]. Journal of the Less Common Metals, 1985, 113(2): 331–339. doi: 10.1016/0022-5088(85)90289-9 [23] WATANABE K, SAKAIRI M, TAKAHASHI H, et al. Formation of Al-Zr composite oxide films on aluminum by sol-gel coating and anodizing [J]. Journal of Electroanalytical Chemistry, 1999, 473(1/2): 250–255. [24] PERDEW J P, RUZSINSZKY A, CSONKA G I, et al. Restoring the density-gradient expansion for exchange in solids and surfaces [J]. Physical Review Letters, 2008, 100(13): 136406. doi: 10.1103/PhysRevLett.100.136406 [25] VANDERBILT D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism [J]. Physical Review B, 1990, 41(11): 7892–7895. doi: 10.1103/PhysRevB.41.7892 [26] MONKHORST H J, PACK J D. Special points for Brillouin-zone integrations [J]. Physical Review B, 1976, 13(12): 5188–5192. doi: 10.1103/PhysRevB.13.5188 [27] VAN DE WALLE C G, NEUGEBAUER J. First-principles calculations for defects and impurities: applications to Ⅲ-nitrides [J]. Journal of Applied Physics, 2004, 95(8): 3851–3879. doi: 10.1063/1.1682673 [28] WU Z J, ZHAO E J, XIANG H P, et al. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles [J]. Physical Review B, 2007, 76(5): 054115. doi: 10.1103/PhysRevB.76.054115 [29] HILL R. The elastic behaviour of a crystalline aggregate [J]. Proceedings of the Physical Society: Section A, 1952, 65(5): 349–354. doi: 10.1088/0370-1298/65/5/307 [30] CHEN X Q, NIU H Y, LI D Z, et al. Modeling hardness of polycrystalline materials and bulk metallic glasses [J]. Intermetallics, 2011, 19(9): 1275–1281. doi: 10.1016/j.intermet.2011.03.026 [31] PUGH S F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals [J]. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 1954, 45(367): 823–843. doi: 10.1080/14786440808520496 [32] SILVI B, SAVIN A. Classification of chemical bonds based on topological analysis of electron localization functions [J]. Nature, 1994, 371(6499): 683–686. doi: 10.1038/371683a0 -






 下载:
下载: