Raman Scattering of Grossular-Andradite Solid Solution
-
摘要: 为了研究Al3+-Fe3+离子替代对固溶体结构的影响,进行了钙铝榴石-钙铁榴石固溶体的拉曼光谱研究。结果表明:钙铝榴石、钙铁榴石端元分别观测到20和19个拉曼峰,多数峰位随成分呈连续、线性变化,光谱中未发现双模式振动;中频峰峰位变化较大,可能与结构联动或耦合振动有关;由于结构对称性降低,振动光谱中发现了额外峰;拉曼峰宽化现象与有序度降低及结构的畸变有关。通过单参数的Margules方程拟合半峰宽,预测了固溶体的混合焓特征。Abstract: The effects of Al3+-Fe3+ substitution on 10 synthesized garnet samples along the grossular-andradite binary were investigated using Raman spectroscopy. Twenty and nineteen peaks were observed in non-polarized Raman spectra for grossular and andradite end-members, respectively. The frequencies of most peaks were changed almost linearly with the composition. Two-mode behavior was not observed in this study. Differing from previous reports on other garnet solid solutions, the medium frequency modes, which are assigned to internal bending vibrations, have the largest average rate of change with the composition, which may be related to structural connectivity and coupled vibrations. Due to the reduction of symmetry, extra peaks appear in the Raman spectra of garnets with intermediate compositions. Peak broadening in intermediate compositions was also observed, which is related to disordering and distortion. One-parameter Margules equation was used to describe the full width at half maximum of peaks, and a relationship with enthalpy of mixing was proposed.
-
Key words:
- grossular-andradite /
- Raman spectroscopy /
- bending vibration /
- extra peak /
- enthalpy of mixing
-
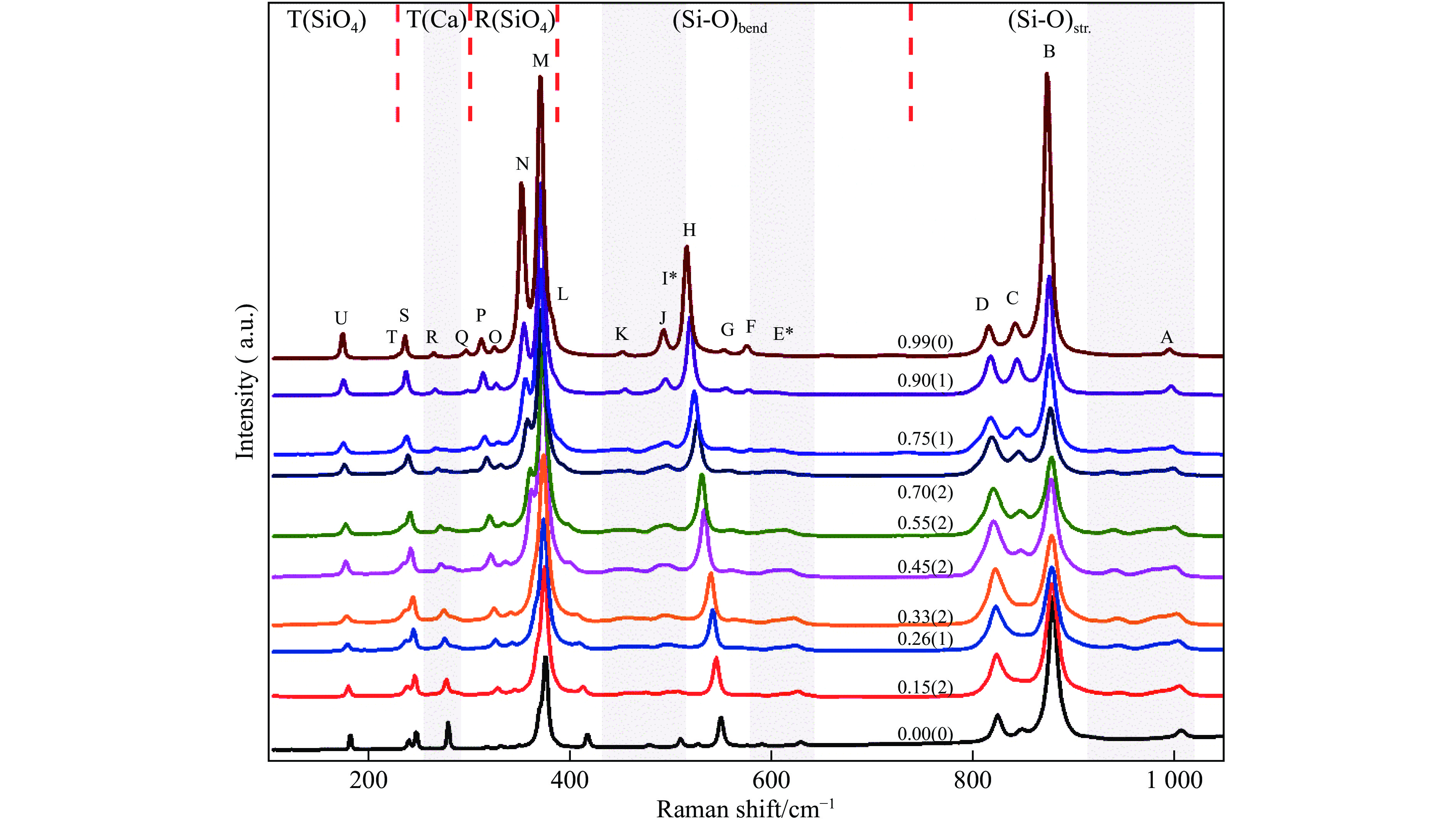
图 1 钙铝榴石-钙铁榴石固溶体的拉曼光谱(光谱分为5个区域[14, 17],数字表示钙铁榴石含量,标注星号的峰未在钙铁榴石端元中发现,灰色区域为额外峰出现位置)
Figure 1. Raman spectra of grossular-andradite solid solutions (The spectra are divided into 5 regions[14, 17]. The number represents the mole fraction of andradite component. The peaks with asterisk can’t be observed for andradite end-member. Grey areas mark the zones where extra peaks are observed.)
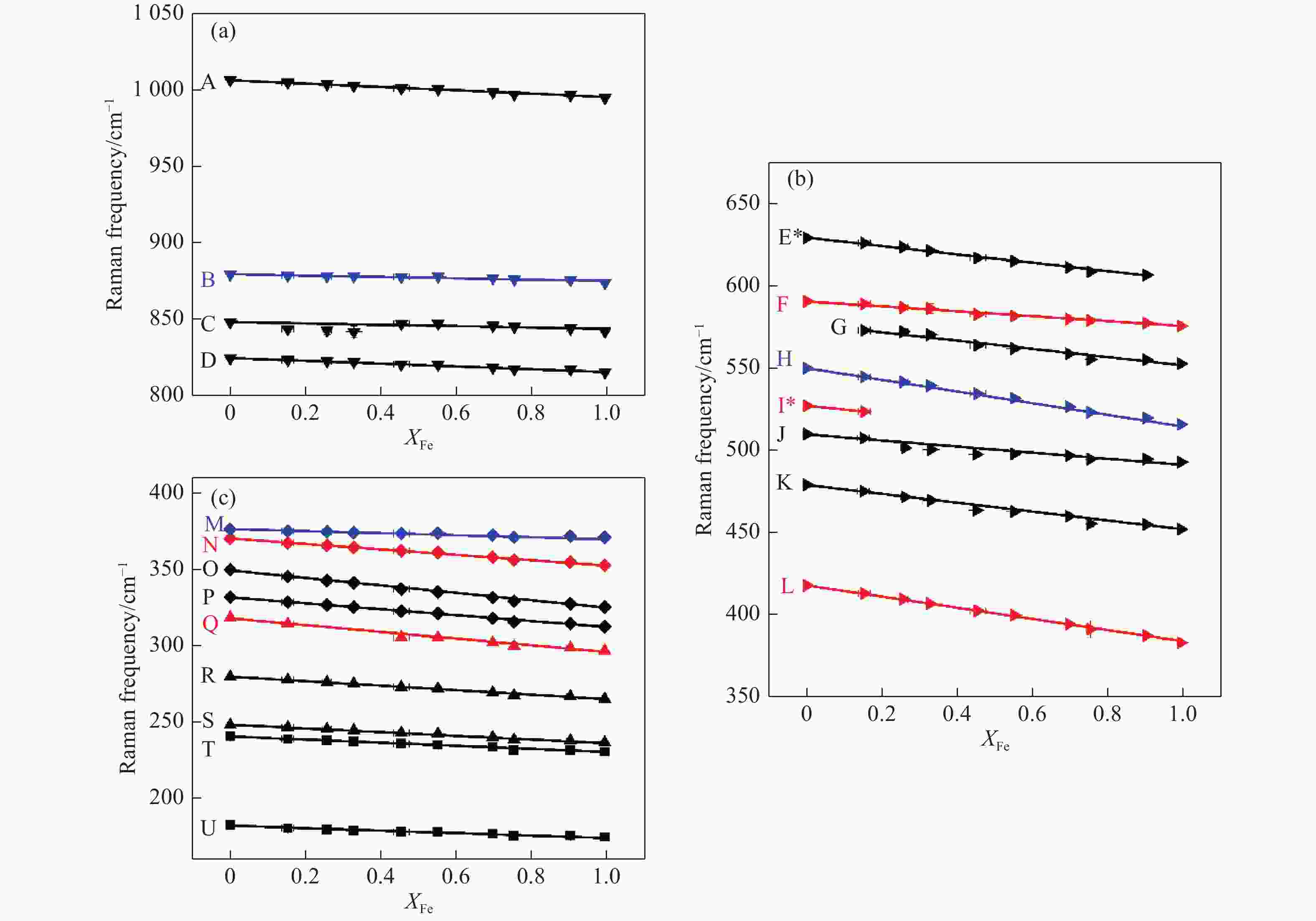
图 2 钙铝榴石-钙铁榴石固溶体的拉曼峰频率-成分变化关系:(a)高频振动模式(Si-O)str.,(b)中频振动模式(Si-O)bend,(c)低频(晶格)振动模式(部分振动模式在固溶体系列中表现不连续性。倒三角为(Si-O)str.,右三角为(Si-O)bend,菱形为R(SiO4),正三角为T(Ca),正方形为T(SiO4)。黑色表示T2g,红色表示Eg,蓝色表示A1g。所有峰均采用最小二乘法拟合。y轴采用相同比例尺,以便对比不同振动模式受离子替代的影响程度。)
Figure 2. Raman frequencies of grossular-andradite solid solution as a function of composition: (a) high frequency (Si-O)str. modes, (b) medium frequency (Si-O)bend modes, (c) low frequency lattice modes.(Several modes show discontinuity along solid solutions. Symbols: downward pointing triangle = (Si-O)str., rightward pointing triangle = (Si-O)bend, diamonds = R(SiO4), upward pointing triangles = T(Ca), squares = T(SiO4). Colors: black = T2g, red = Eg, blue = A1g. A linear regression by least-squares analyses was applied to all the peak frequencies. Note that the y axes have the same scales and the steepness of the slopes can be compared between the three plots.)
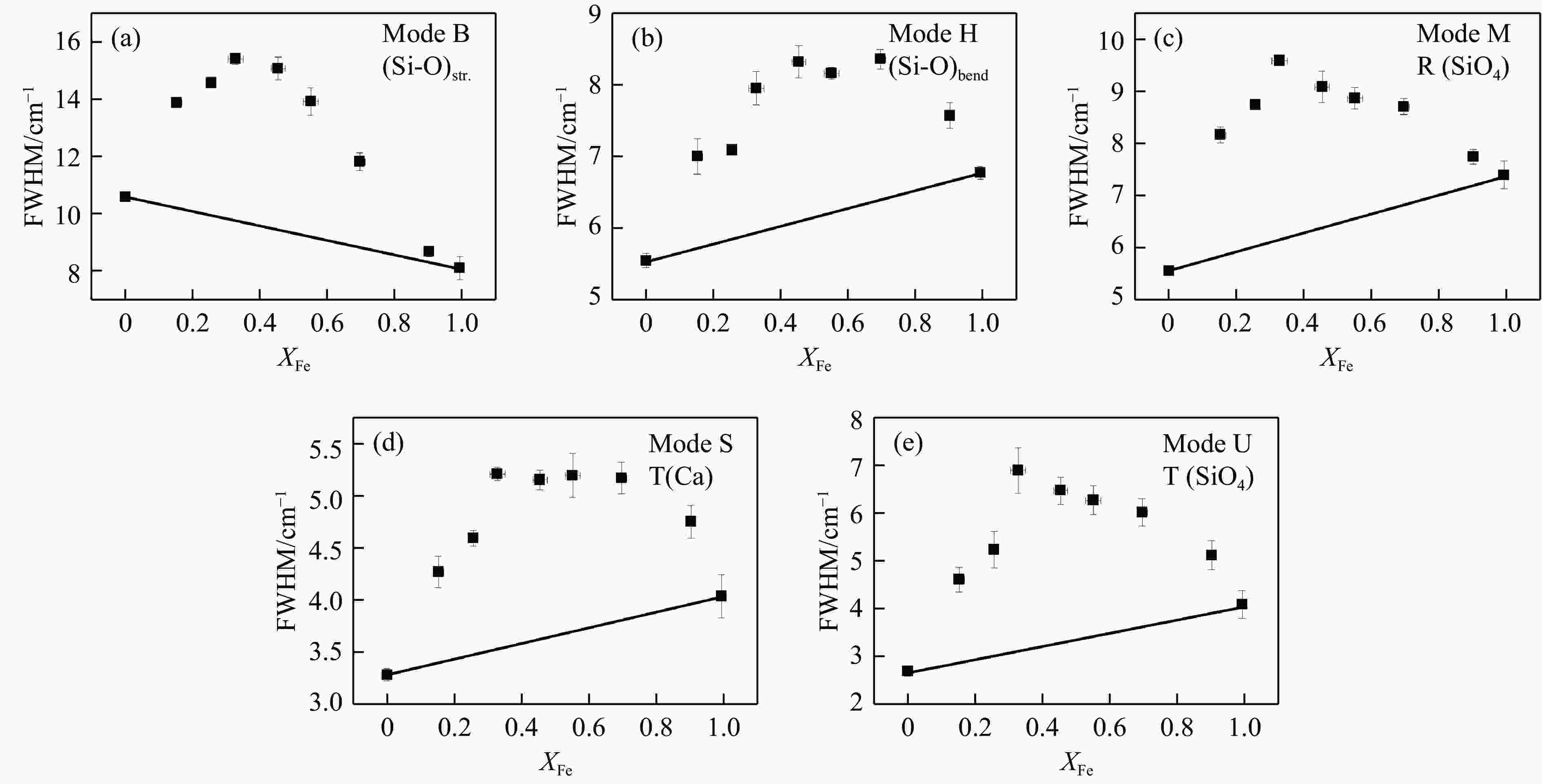
图 3 钙铝榴石-钙铁榴石固溶体的半峰宽-成分变化,5个拉曼峰分别归属于[14]:(a) Si-O伸缩振动,(b) Si-O弯曲振动,(c) SiO4转动,(d) Ca平动,(e) SiO4平动
Figure 3. Peak widths of selected Raman modes change with composition along grossular-andradite solid solution. The vibrations these peaks can be assigned to Kolesov, et al.[14] are: Si-O stretching (a), Si-O bending (b), SiO4 rotation (c), Ca translation (d) and SiO4 translation (e)
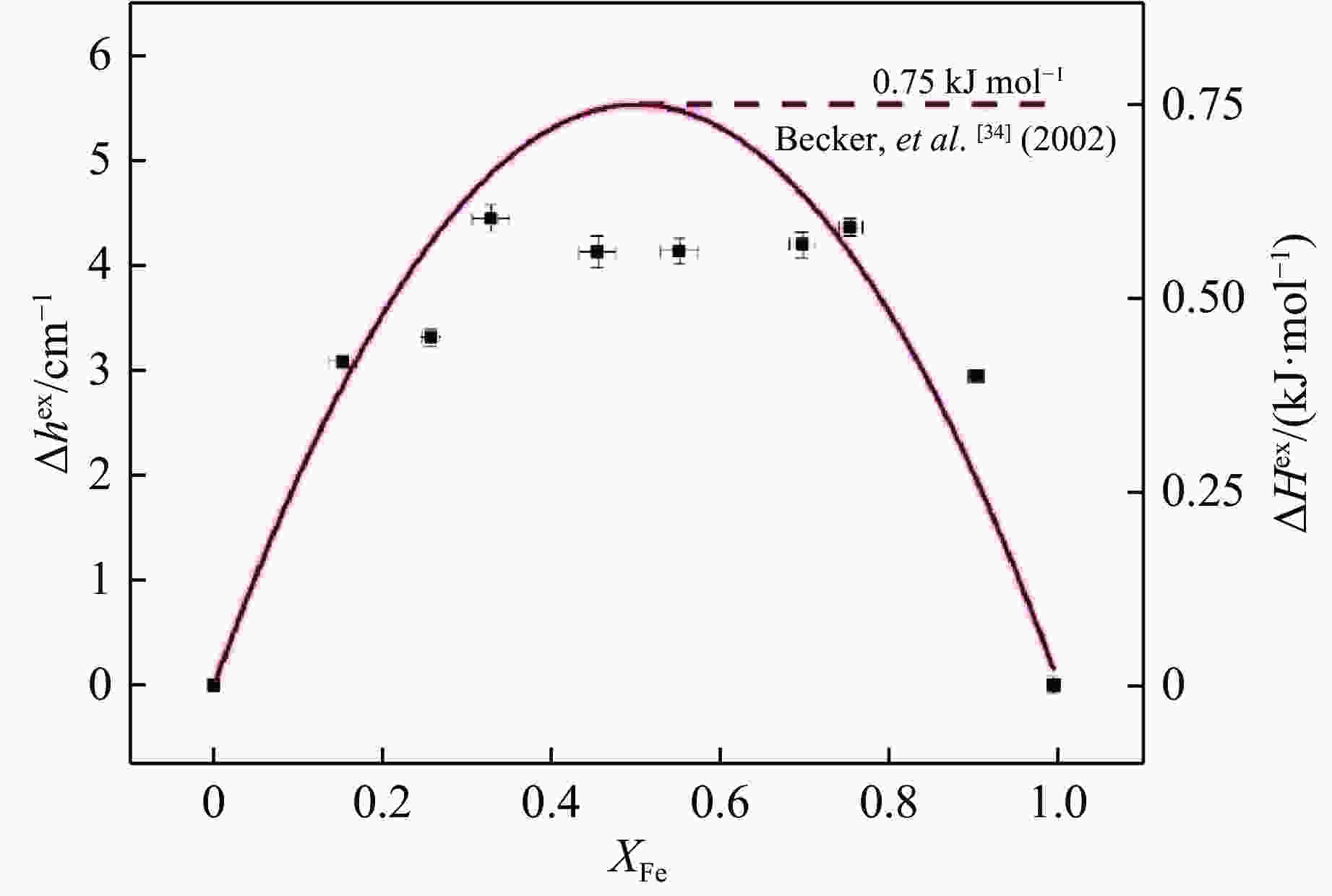
图 5 镁铝榴石-钙铝榴石固溶体的超额半峰宽(
$\Delta {h^{{\rm{ex}}}}$ )拟合曲线(黑色虚线为Du等[13]的L峰数据,红色点线为预测超额混合焓($\Delta H_{{\rm{FWHM}}}^{{\rm{ex}}}$ )曲线,红色实线为超额混合焓测热数据($\Delta H_{{\rm{Cal}}}^{{\rm{ex}}}$ )拟合曲线[10])Figure 5. Fitting curve of
$\Delta {h^{{\rm{ex}}}}$ for pyrope-grossular solid solution (Black dashed line: peak L of Du, et al.[13] Red dotted line: the predicted excess enthalpy of mixing curve. Red solid line: the fitting curve for calorimetric excess enthalpy of mixing data from Newton, et al.[10])表 1 钙铝榴石-钙铁榴石固溶体拉曼频率的最小二乘法拟合结果
Table 1. Linear regression results of Raman frequencies of Gro-And solid solution versus composition
Peak No. ν0/cm−1 Slope/(cm−1·mol−1% ) Error Assign AVG/(cm−1·Å−1) R2 A 1007 −10.96 0.42 (Si-O)str. −34.67 0.99 B 880 −4.44 0.35 0.95 C 848 −4.49 0.89 0.73 D 825 −9.22 0.51 0.97 E 629 −25.49 0.85 (Si-O)bend −121.52 0.99 F 591 −14.95 0.22 1.00 G −25.11 2.12 0.95 H 550 −35.54 0.40 1.00 I 527 −23.42 J 510 −18.60 0.79 0.98 K 479 −27.02 0.41 1.00 L 418 −34.02 0.26 1.00 M 376 −6.64 0.77 R(SiO4) −81.33 0.89 N 370 −17.81 0.47 0.99 O 350 −24.42 0.48 1.00 P 332 −19.43 0.30 1.00 Q 318 −22.07 0.99 T(Ca) −77.76 0.99 R 279 −14.72 0.25 1.00 S 248 −12.20 0.55 0.98 T 240 −10.14 0.17 T(SiO4) −43.48 1.00 U 182 −8.13 0.54 0.96 Note: (1) The Raman modes of Gro and And are assigned according to Kolesov, et al[14].
(2) AVG:average value of slopes in each assignment. Unit cell parameters of grossular and andradite are 11.84 Å and 12.05 Å respectively according to our single crystal XRD data. -
[1] GREW E S, LOCOCK A J, MILLS S J, et al. Nomenclature of the garnet supergroup [J]. American Mineralogist, 2013, 98(4): 785–810. doi: 10.2138/am.2013.4201 [2] HAZEN R M, DOWNS R T, CONRAD P G, et al. Comparative compressibilities of majorite-type garnets [J]. Physics and Chemistry of Minerals, 1994, 21(5): 344–349. doi: 10.1007/BF00202099 [3] CONRAD P G, ZHA C S, MAO H K, et al. The high-pressure, single-crystal elasticity of pyrope, grossular, and andradite [J]. American Mineralogist, 1999, 84(3): 374–383. doi: 10.2138/am-1999-0321 [4] DU W, CLARK S M, WALKER D. Thermo-compression of pyrope-grossular garnet solid solutions: non-linear compositional dependence [J]. American Mineralogist, 2015, 100(1): 215–222. doi: 10.2138/am-2015-4752 [5] BLUNDY J, WOOD B. Prediction of crystal-melt partition coefficients from elastic moduli [J]. Nature, 1994, 372(6505): 452–454. doi: 10.1038/372452a0 [6] SUN C G, LIANG Y. The importance of crystal chemistry on REE partitioning between mantle minerals (garnet, clinopyroxene, orthopyroxene, and olivine) and basaltic melts [J]. Chemical Geology, 2013, 358: 23–36. doi: 10.1016/j.chemgeo.2013.08.045 [7] GANGULY J, CHENG W J, O’NEILL H S C. Syntheses, volume, and structural changes of garnets in the pyrope-grossular join: implications for stability and mixing properties [J]. American Mineralogist, 1993, 78(5/6): 583–593. [8] GEIGER C A. Silicate garnet: a micro to macroscopic (re)view [J]. American Mineralogist, 2008, 93(2/3): 360–372. doi: 10.2138/am.2008.2588 [9] GEIGER C A, FEENSTRA A. Molar volumes of mixing of almandine-pyrope and almandine-spessartine garnets and the crystal chemistry and thermodynamic-mixing properties of the aluminosilicate garnets [J]. American Mineralogist, 1997, 82(5/6): 571–581. doi: 10.2138/am-1997-5-617 [10] NEWTON R C, CHARLU T V, KLEPPA O J. Thermochemistry of high pressure garnets and clinopyroxenes in the system CaO-MgO-Al2O3-SiO2 [J]. Geochimica et Cosmochimica Acta, 1977, 41(3): 369–377. doi: 10.1016/0016-7037(77)90264-2 [11] UNGARETTI L, LEONA M, MERLI M, et al. Non-ideal solid-solution in garnet: crystal-structure evidence and modelling [J]. European Journal of Mineralogy, 1995, 7(6): 1299–1312. doi: 10.1127/ejm/7/6/1299 [12] BOFFA BALLARAN T, CARPENTER M A, GEIGER C A, et al. Local structural heterogeneity in garnet solid solutions [J]. Physics and Chemistry of Minerals, 1999, 26(7): 554–569. doi: 10.1007/s002690050219 [13] DU W, HAN B F, CLARK S M, et al. Raman spectroscopic study of synthetic pyrope-grossular garnets: structural implications [J]. Physics and Chemistry of Minerals, 2018, 45(2): 197–209. doi: 10.1007/s00269-017-0908-z [14] KOLESOV B A, GEIGER C A. Raman spectra of silicate garnets [J]. Physics and Chemistry of Minerals, 1998, 25(2): 142–151. doi: 10.1007/s002690050097 [15] MCALOON B P, HOFMEISTER A M. Single-crystal IR spectroscopy of grossular-andradite garnets [J]. American Mineralogist, 1995, 80(11/12): 1145–1156. doi: 10.2138/am-1995-11-1205 [16] HOFMEISTER A M, FAGAN T J, CAMPBELL K M, et al. Single-crystal IR spectroscopy of pyrope-almandine garnets with minor amounts of Mn and Ca [J]. American Mineralogist, 1996, 81(3/4): 418–428. doi: 10.2138/am-1996-3-416 [17] HOFMEISTER A M, CHOPELAS A. Vibrational spectroscopy of end-member silicate garnets [J]. Physics and Chemistry of Minerals, 1991, 17(6): 503–526. doi: 10.1007/BF00202230 [18] SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides [J]. Acta Crystallographica Section A, 1976, 32(5): 751–767. doi: 10.1107/S0567739476001551 [19] BOFFA BALLARAN T, WOODLAND A B. Local structure of ferric iron-bearing garnets deduced by IR-spectroscopy [J]. Chemical Geology, 2006, 225(3/4): 360–372. doi: 10.1016/j.chemgeo.2005.08.028 [20] WANG Y C, SUN Q, DUAN D F, et al. The study of crystal structure on grossular-andradite solid solution [J]. Minerals, 2019, 9(11): 691. doi: 10.3390/min9110691 [21] LIU X, CHEN J L, TANG J J, et al. A large volume cubic press with a pressure-generating capability up to about 10 GPa [J]. High Pressure Research, 2012, 32(2): 239–254. doi: 10.1080/08957959.2012.657634 [22] GILLET P, FIQUET G, MALEZIEUX J M, et al. High-pressure and high-temperature Raman spectroscopy of end-member garnets: pyrope, grossular and andradite [J]. European Journal of Mineralogy, 1992, 4(4): 651–664. doi: 10.1127/ejm/4/4/0651 [23] MOORE R K, WHITE W B, LONG T V. Vibrational spectra of the common silicates: I. the garnets [J]. American Mineralogist, 1971, 56(1/2): 54–71. [24] PASCALE F, CATTI M, DAMIN A, et al. Vibration frequencies of Ca3Fe2Si3O12 andradite: an ab initio study with the CRYSTAL code [J]. Journal of Physical Chemistry B, 2005, 109(39): 18522–18527. doi: 10.1021/jp052991e [25] PASCALE F, ZICOVICH-WILSON C M, ORLANDO R, et al. Vibration frequencies of Mg3Al2Si3O12 pyrope. an ab initio study with the CRYSTAL code [J]. The Journal of Physical Chemistry B, 2005, 109(13): 6446–6152. doi: 10.1021/jp050316z [26] BORN L, ZEMANN J. Abstandsberechnungen und gitterenergetische Berechnungenan Granaten [J]. Beiträgezur Mineralogie und Petrographie, 1964, 10(1): 2–23. doi: 10.1007/BF01192531 [27] WOODLAND A B, ROSS II C R. A crystallographic and mössbauer spectroscopy study of ${ {\rm{Fe}}_3^{2 + } }$ Al2Si3O12-${{\rm{Fe}}_3^{2 + }{\rm{Fe}}_2^{3 + }}$ Si3O12, (Almandine-"Skiagite") and Ca3${{\rm{Fe}}_3^{2 + }}$ Si3O12-${{\rm{Fe}}_3^{2 + }{\rm{Fe}}_2^{3 + }}$ Si3O12 (Andradite-"Skiagite") garnet solid solutions [J]. Physics and Chemistry of Minerals, 1994, 21(3): 117–132. doi: 10.1007/BF00203142[28] ZIMAN J M. Models of disorder: the theoretical physics of homogeneously disordered systems [M]. Cambridge: Cambridge University Press, 1979. [29] DE LA PIERRE M, NOEL Y, MUSTAPHA S, et al. The infrared vibrational spectrum of andradite-grossular solid solutions: a quantum mechanical simulation [J]. American Mineralogist, 2013, 98(5/6): 966–976. doi: 10.2138/am.2013.4156 [30] DEMPSEY M J. Evidence for structural changes in garnet caused by calcium substitution [J]. Contributions to Mineralogy and Petrology, 1980, 71(3): 281–282. doi: 10.1007/BF00371669 [31] FEI X H, ZHANG Z C, CHENG Z G, et al. Factors controlling the crystal morphology and chemistry of garnet in skarn deposits: a case study from the Cuihongshan polymetallic deposit, Lesser Xing’an Range, NE China [J]. American Mineralogist, 2019, 104(10): 1455–1468. doi: 10.2138/am-2019-6968 [32] GASPAR M, KNAACK C, MEINERT L D, et al. REE in skarn systems: a LA-ICP-MS study of garnets from the Crown Jewel gold deposit [J]. Geochimica et Cosmochimica Acta, 2008, 72(1): 185–205. doi: 10.1016/j.gca.2007.09.033 [33] XU J, CIOBANU C L, COOK N J, et al. Skarn formation and trace elements in garnet and associated minerals from Zhibula copper deposit, Gangdese Belt, southern Tibet [J]. Lithos, 2016, 262: 213–231. doi: 10.1016/j.lithos.2016.07.010 [34] BECKER U, POLLOK K. Molecular simulations of interfacial and thermodynamic mixing properties of grossular-andradite garnets [J]. Physics and Chemistry of Minerals, 2002, 29(1): 52–64. doi: 10.1007/s002690100211 -






 下载:
下载: