High Pressure Raman Spectroscopy and X-ray Diffraction of CuS2
-
摘要: 利用金刚石压腔在高温高压条件下合成了黄铁矿结构CuS2,结合显微拉曼光谱和同步辐射X射线衍射实验,发现黄铁矿结构CuS2在低于30 GPa的压力范围内保持稳定,无结构相变发生。高压拉曼光谱研究结果显示,CuS2的所有拉曼模频率随压力升高呈连续单调线性增加。对X射线衍射实验获得的CuS2体积随压力变化关系进行Birch-Murnaghan状态方程拟合,得到零压晶胞体积V0 = 193.8(5)Å3,体积模量K0 = 99(2)GPa,K0'=4(固定)。运用第一性原理理论计算,得到CuS2的拉曼模频率和晶胞体积随压力变化关系均与实验观测结果保持一致。与其他黄铁矿结构过渡金属二硫化物MS2(M = Mn,Fe,Co,Ni)对比,发现MS6配位八面体大小(M—S键长)主导晶胞的体积大小和晶体的压缩性,并推测CuS2中Cu离子可能以+2价形式存在。研究结果弥补了黄铁矿结构CuS2高压拉曼光谱和X射线衍射研究的缺失,证实其在高温高压下的结构稳定性,对全面认识CuS2的物理化学性质及黄铁矿结构物质的统一规律具有重要价值,对于探索Cu在地球深部的价态和赋存形式也具有指示意义。Abstract: Pyrite structure CuS2 was synthesized in diamond anvil cell at high pressures and high temperatures. Using Raman spectroscopy and synchrotron X-ray diffraction, the pyrite-type CuS2 was found to be stable in 0-30 GPa without any phase transition. Raman spectroscopy show that all observed Raman frequencies increasemonotonously with increasing pressures. Fitting experimental pressure and volume data of X-ray diffraction with Birch-Murnaghan equation of state, gives V0 = 193.8(5) Å3, K0 = 99(2) GPa and K0' = 4 (fix). The dependencies of Raman frequencies and unit-cell volumes with pressures are coincident with the results of first-principles calculation. The results of calculation properly depict that of experiments. Compared with other pyrite structure transition-metal disulfides MS2(M = Mn, Fe, Co, Ni), the length of M—S dominates the unit-cell volume and compressibility of MS2, and the Cu cation tends to be +2 valance in the CuS2. This study makes up for the lack of high-pressure Raman and XRD research of CuS2, and confirms structural stability of pyrite-type CuS2 at high pressures and high temperatures. The results are important for comprehending the physical and chemical properties of CuS2 and realizing the unified law of pyrite structure materials. It’s also meaningful in discussion of the valance and distribution of copper in deep Earth.
-
Key words:
- pyrite structure /
- CuS2 /
- Raman spectroscopy /
- X-ray diffraction /
- high pressure
-
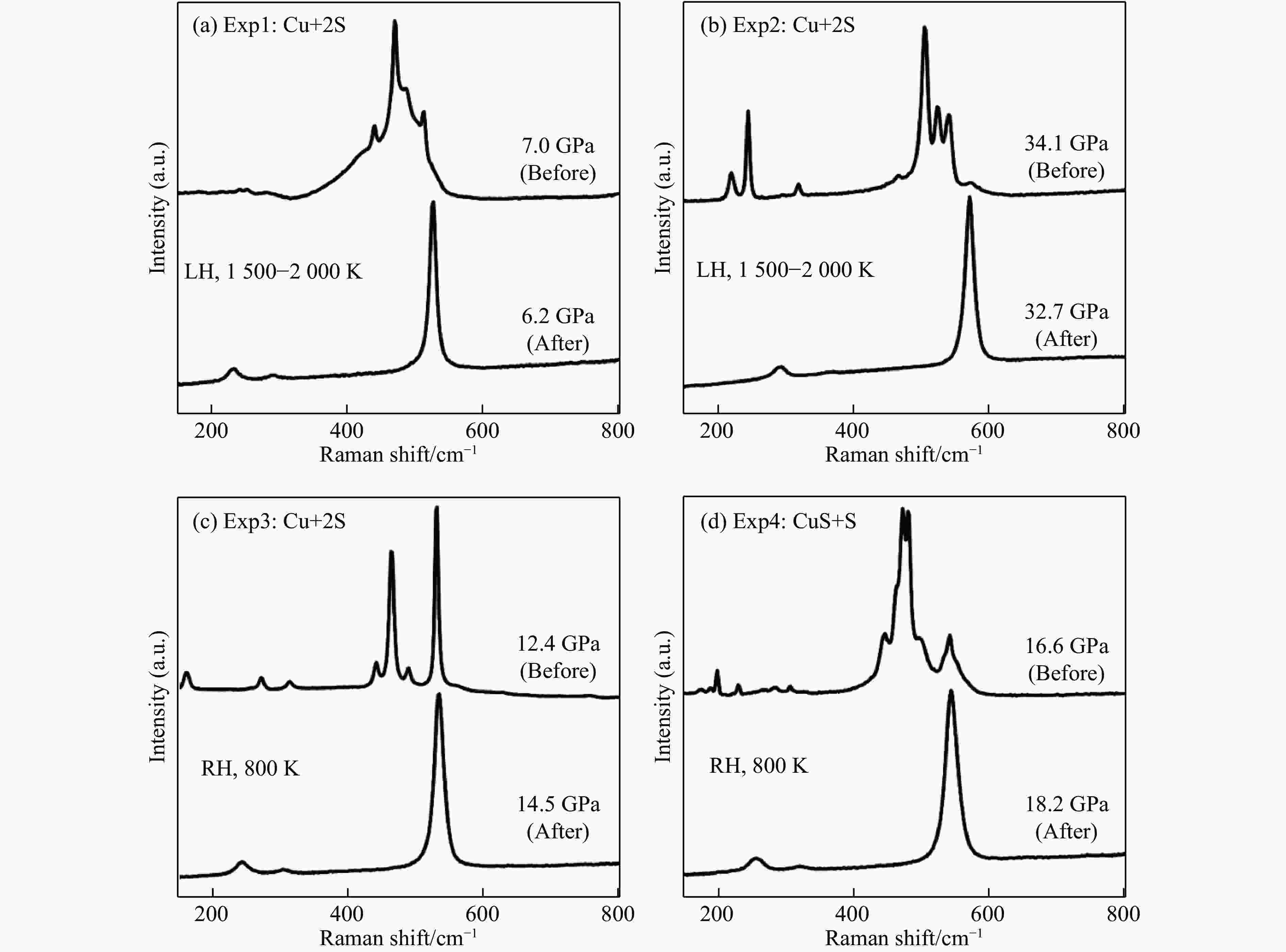
图 2 不同条件下4组实验反应前后的特征拉曼光谱(LH和RH分别代表激光加温和电阻丝加温,4组实验中加温前所测拉曼信号均为单质硫的高压相,加温后产物为黄铁矿结构CuS2)
Figure 2. Raman spectra of the four experiments at different conditions before and after reaction (LH and RH represent laser heating and resistance heating, respectively. Before heating, all the Raman peaks in four experiments belong to the high-pressure phase of elemental sulfur. After heating, the reaction products are pyrite structure CuS2.)
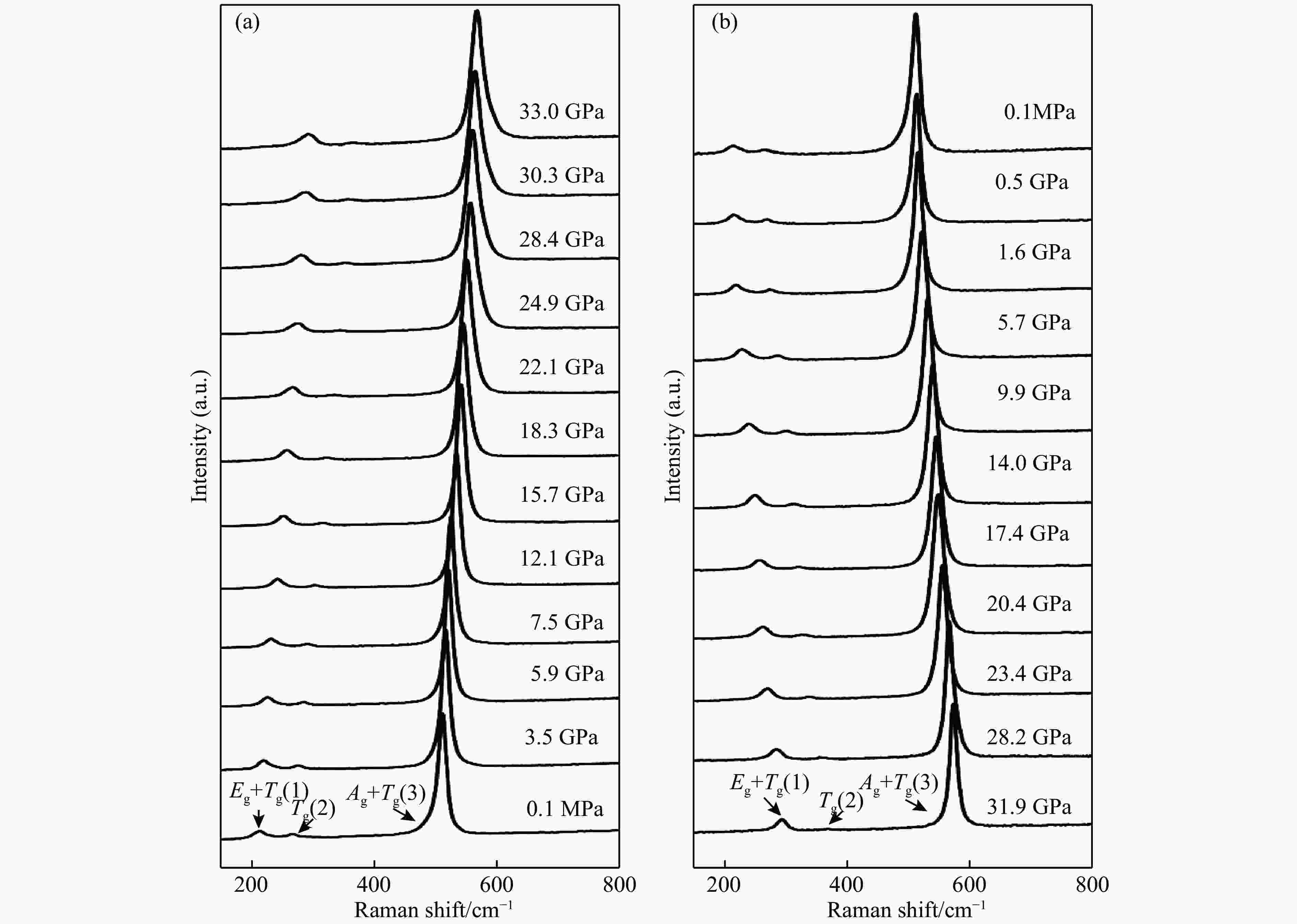
图 4 实验和理论计算CuS2的拉曼频率随压力变化关系(黑色圆形和菱形分别对应Exp4合成的CuS2在常温加压和卸压过程的结果,三角形表示Exp2合成CuS2卸压过程的数据,星形为理论计算值,实线和虚线分别为实验和计算数据线性拟合结果)
Figure 4. Experimental and theory calculated pressure dependence of Raman vibrational modes of CuS2(Black spheres and diamonds correspond to the results of compression process and decompression process of CuS2 synthesised at Exp4,respectively. Black triangles are data of decompression process of CuS2 synthesised at Exp2. The stars represent theory calculated points. Solid line and dotted line are linnear fitted with all experimental and calculated data, respectively.)
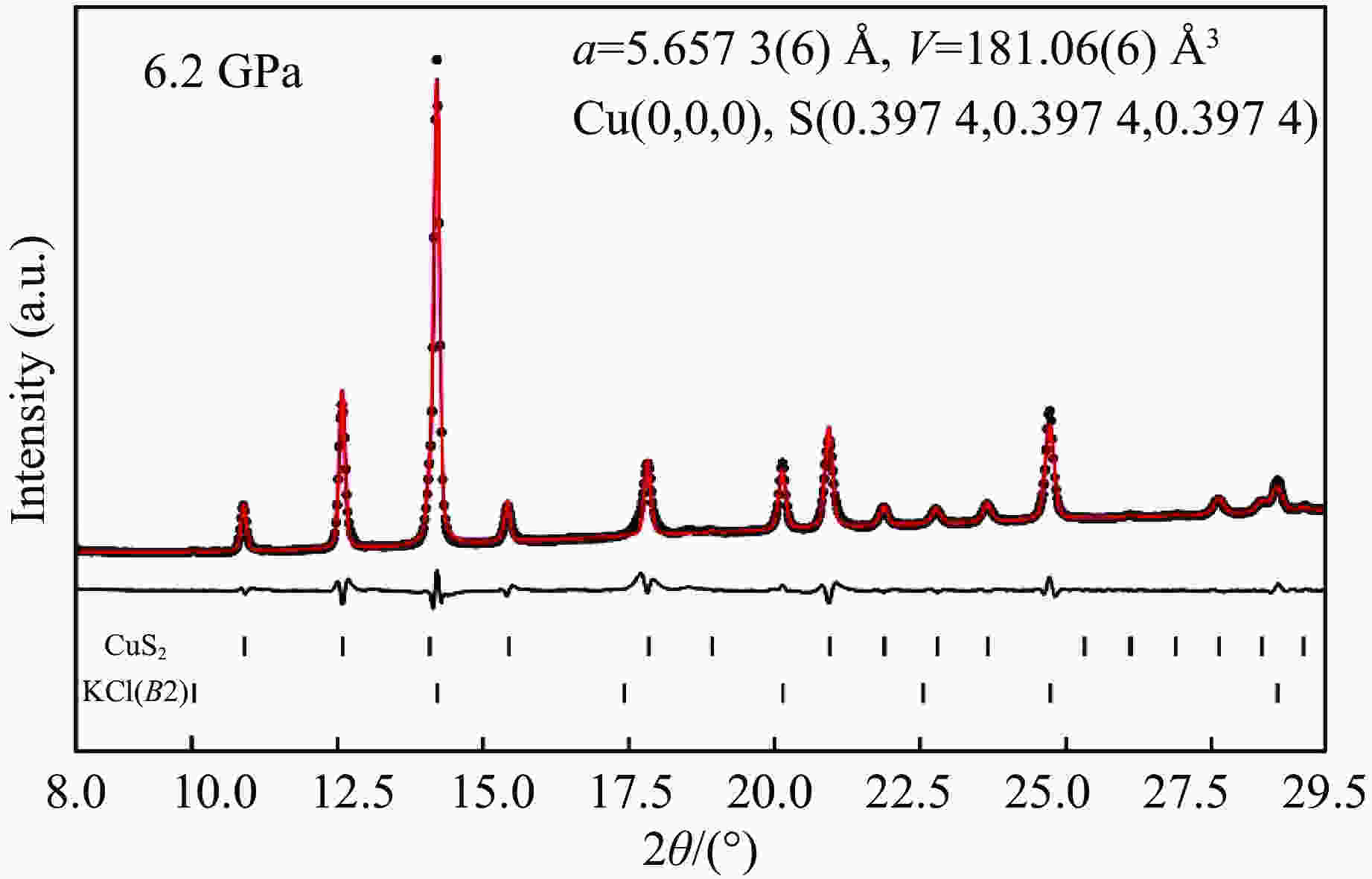
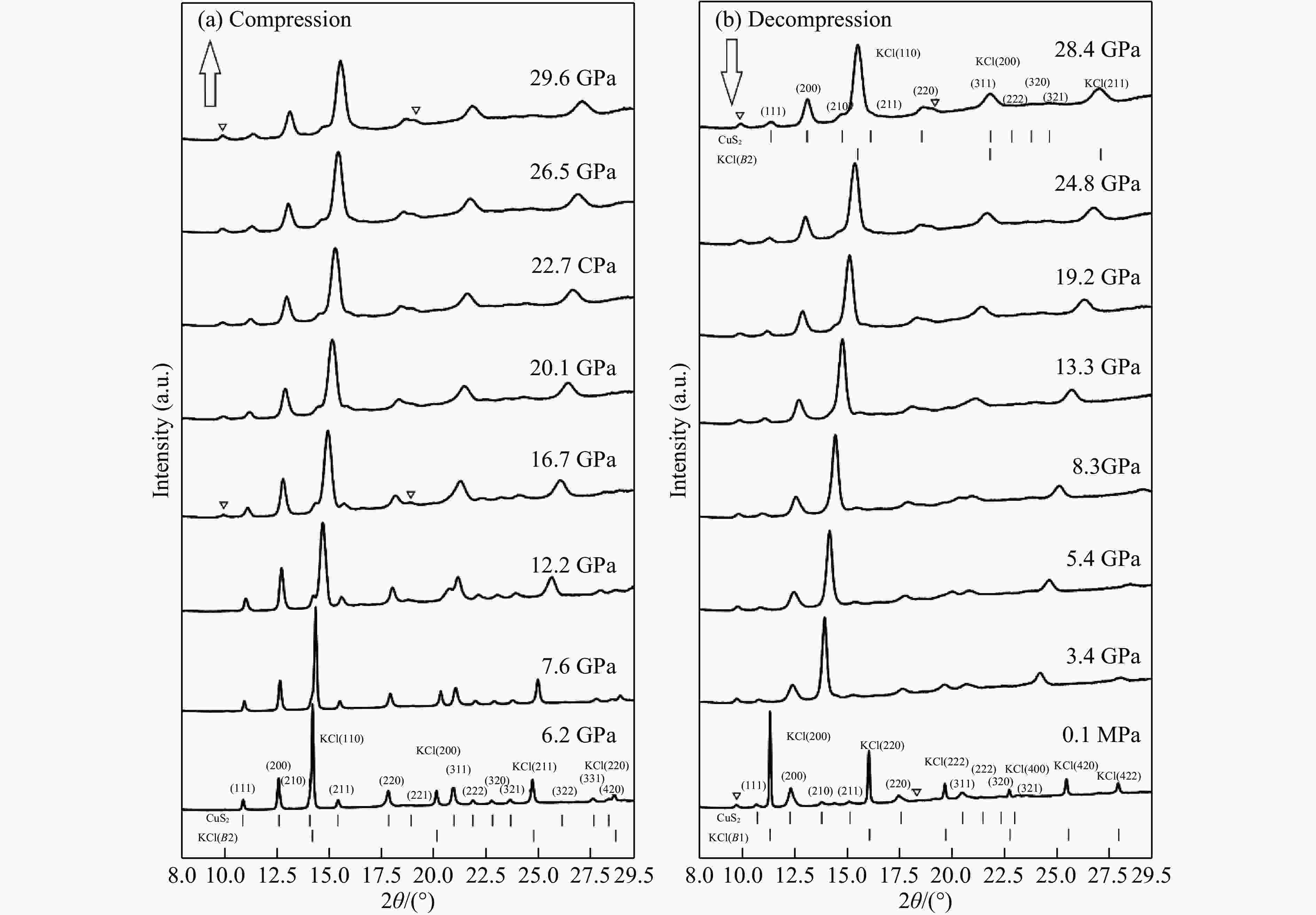
图 6 在加压(a)和卸压(b)过程中CuS2在不同压力下的X射线衍射图谱(下三角指示产物中的杂质峰,黄铁矿结构CuS2和传压介质KCl的衍射峰位在图中用短棒标出)
Figure 6. X-ray powder diffraction patterns at different pressures on compression (a) and decompression (b)(The inverted triangles indicate impurity peaks. The diffraction peaks of pyrite structure CuS2 and pressure transmitting medium KCl are represented with vertical bars.)
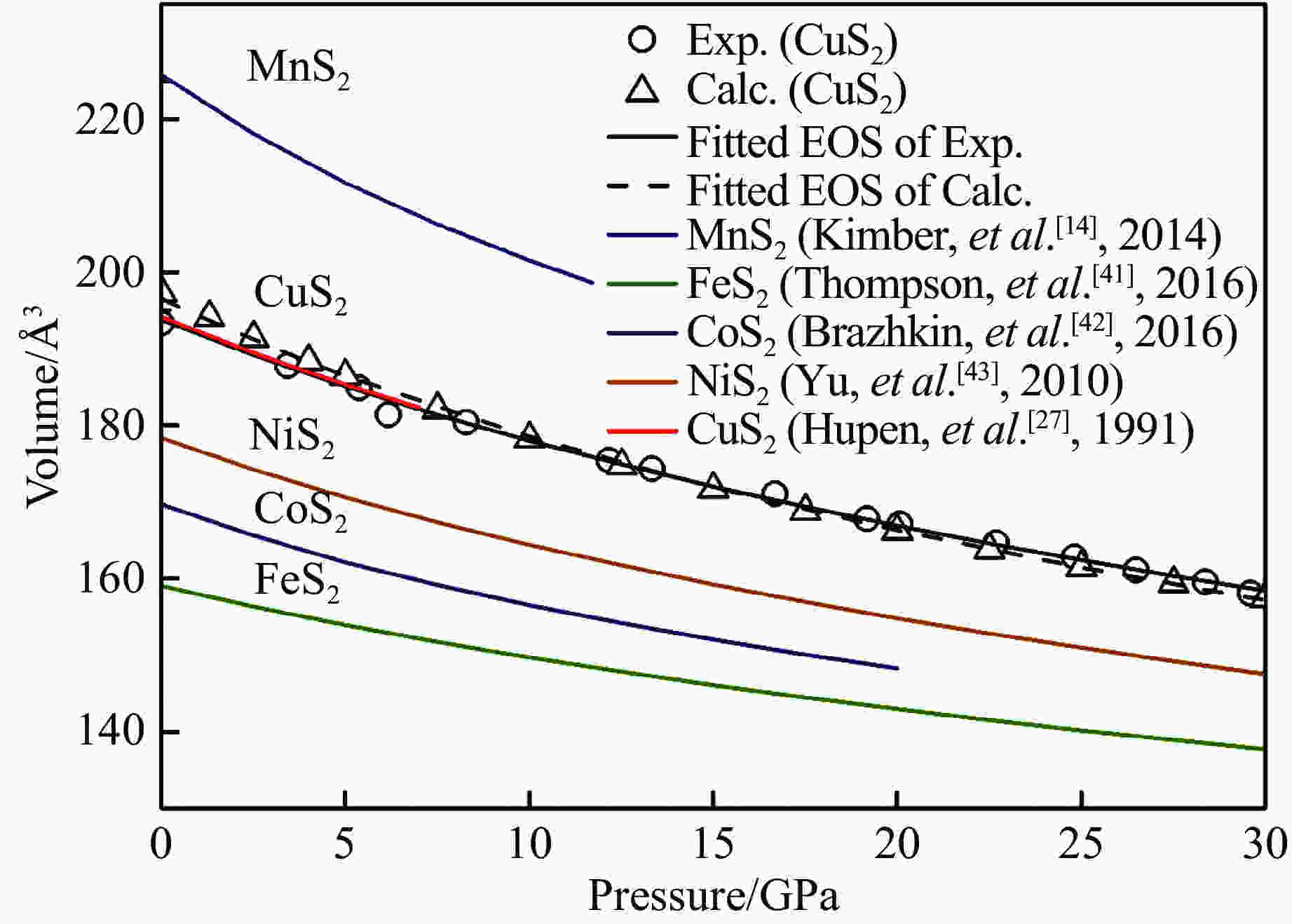
图 7 理论计算和衍射实验的CuS2及其它过渡金属二硫化物MS2(M = Mn,Fe,Co,Ni,Cu)的体积-压力变化关系(黑色圆形和黑色三角形分别对应X射线衍射实验和第一性原理理论计算结果。黑色实线和黑色虚线是对实验和计算数据进行Birch-Murnaghan 状态方程拟合结果。蓝色、绿色、紫色、棕色和红色实线分别为文献给出的黄铁矿结构MnS2、FeS2、CoS2、NiS2和CuS2状态方程结果。)
Figure 7. Pressure-volume relationship of experimental and calculated CuS2 and other transition metal disulfides MS2(M = Mn,Fe,Co,Ni,Cu) (The black circles and triangles represent the experimental and observed data of the CuS2, respectively. The solid black line and dotted black line are the Birch-Murnaghan equation of state fit respectively with listed parameters. The blue, green, purple, yellow, and red line shows the equation of state of the pyrite structure MnS2, FeS2, CoS2, NiS2, and CuS2 in references.)
表 1 黄铁矿结构CuS2的拉曼频率随压力变化及格临爱森参数(γ)
Table 1. Pressure dependences of Raman modes and the Grüneisen parameters (γ) of pyrite-type CuS2
Symmetry classification ω0/cm−1 (dω/dp)/(cm−1·GPa−1) γ Method This work Ref.[38] Eg + Tg(1) 213 207 2.45 1.14 Exp. Tg(2) 266 264 3.06 1.14 Ag + Tg(3) 512 512 1.75 0.34 Tg(1) 206 2.33 0.97 Calc. Eg 207 2.31 0.96 Tg(2) 257 3.11 1.04 Tg(3) 495 1.80 0.31 Ag 499 1.41 0.24 Note: The K0(Exp.) = 99 and K0(Calc.) = 85.6 are used respectively to calculate the Grüneisen parameters. They are obtained from the fitted Birch-Murnaghan EOS in this work. 表 2 黄铁矿结构CuS2与其他黄铁矿结构过渡金属二硫化物的零压体积模量和晶胞体积对比
Table 2. Comparison of zero-pressure bulk modulus (K0) and unit formula volume (V0) of CuS2 with that of other pyrite structure transition-metal disulfides
Compositions Pressure range/GPa V0/Å3 K0/GPa K0′ Reference MnS2 0−11.7 225.74(0) 65.9(3) 5.1(2) Ref.[14] FeS2 0−80 159.00(7) 140.2(15) 5.52(19) Ref.[41] CoS2 0−20 169.68(1) 94(2) 6.9(5) Ref.[42] NiS2 0−150 178.32 102.1 4.6 Ref.[43] CuS2 0−7 194.10(10) 98.8(6) 4(fix) Ref.[27] CuS2 0−29.6 193.8(5) 99(2) 4(fix) This study (Exp.) CuS2 0−30 196.5(2) 85.6(7) 4(fix) This study (Calc.) Note: All MS2(M = Mn,Fe,Co,Ni,Cu) are pyrite structure (Pa3) in p-range. All the V0 are uniformly transformed to same units for comparison purposes. -
[1] BROSTIGEN G, KJEKSHUS A. Redetermined crystal structure of FeS2 (Pyrite) [J]. Acta Chemica Scandinavica, 1969, 23(6): 2186–2188. doi: 10.3891/acta.chem.scand.23-2186 [2] BITHER T A, BOUCHARD R, CLOUD W, et al. Transition metal pyrite dichalcogenides. High-pressure synthesis and correlation of properties [J]. Inorganic Chemistry, 1968, 7(11): 2208–2220. [3] NOWACK E, SCHWARZENBACH D, HAHN T. Charge densities in CoS2 and NiS2 (pyrite structure) [J]. Acta Crystallographica Section B: Structural Science, 1991, 47(5): 650–659. doi: 10.1107/S0108768191004871 [4] MAKOVICKY E. Crystal structures of sulfides and other chalcogenides [J]. Reviews in Mineralogy and Geochemistry, 2006, 61(1): 7–125. doi: 10.2138/rmg.2006.61.2 [5] TEMPLETON D H, DAUBEN C H. The crystal structure of sodium superoxide [J]. Journal of the American Chemical Society, 1950, 72(5): 2251–2254. doi: 10.1021/ja01161a103 [6] KJEKSHUS A, RAKKE T. Preparation and properties of magnesium, copper, zinc and cadmium dichalcogenides [J]. Acta Chemica Scandinavica A, 1979, 33(8): 617–620. doi: 10.3891/acta.chem.scand.33a-0617 [7] KUWAYAMA Y, HIROSE K, SATA N, et al. The pyrite-type high-pressure form of silica [J]. Science, 2005, 309(5736): 923–925. doi: 10.1126/science.1114879 [8] SHIRAKO Y, WANG X, TSUJIMOTO Y, et al. Synthesis, crystal structure, and electronic properties of high-pressure PdF2-type oxides MO2(M= Ru, Rh, Os, Ir, Pt) [J]. Inorganic Chemistry, 2014, 53(21): 11616–11625. doi: 10.1021/ic501770g [9] YU R, ZHAN Q, DE JONGHE L C. Crystal structures of and displacive transitions in OsN2, IrN2, RuN2, and RhN2 [J]. Angewandte Chemie International Edition, 2007, 46(7): 1136–1140. doi: 10.1002/anie.200604151 [10] HU Q, KIM D Y, YANG W, et al. FeO2 and FeOOH under deep lower-mantle conditions and Earth’s oxygen-hydrogen cycles [J]. Nature, 2016, 534(7606): 241–244. doi: 10.1038/nature18018 [11] LIU J, HU Q Y, BI W L, et al. Altered chemistry of oxygen and iron under deep Earth conditions [J]. Nature Communications, 2019, 10(1): 153. doi: 10.1038/s41467-018-08071-3 [12] KLEPPE A K, JEPHCOAT A P. High-pressure Raman spectroscopic studies of FeS2 pyrite [J]. Mineralogical Magazine, 2004, 68(3): 433–441. doi: 10.1180/0026461046830196 [13] HARRAN I, CHEN Y Z, WANG H Y, et al. High-pressure induced phase transition of FeS2: electronic, mechanical and thermoelectric properties [J]. Journal of Alloys and Compounds, 2017, 710: 267–273. doi: 10.1016/j.jallcom.2017.03.256 [14] KIMBER S A J, SALAMAT A, EVANS S R, et al. Giant pressure-induced volume collapse in the pyrite mineral MnS2 [J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(14): 5106–5110. doi: 10.1073/pnas.1318543111 [15] FUJII T, TANAKA K, MARUMO F, et al. Structural behaviour of NiS2 up to 54 kbar [J]. Mineralogical Journal, 1987, 13(7): 448–454. doi: 10.2465/minerj.13.448 [16] ELGHAZALI M A, NAUMOV P G, MU Q, et al. Pressure-induced metallization, transition to the pyrite-type structure, and superconductivity in palladium disulfide PdS2 [J]. Physical Review B, 2019, 100(1): 014507. doi: 10.1103/PhysRevB.100.014507 [17] HUANG S X, WU X, QIN S. Ultrahigh-pressure phase transitions in FeS2 and FeO2: implications for super-earths' deep interior [J]. Journal of Geophysical Research, 2018, 123(1): 277–284. doi: 10.1002/2017JB014766 [18] BITHER T A, PREWITT C T, GILLSON J L, et al. New transition metal dichalcogenides formed at high pressure [J]. Solid State Communications, 1966, 4(10): 533–535. doi: 10.1016/0038-1098(66)90419-4 [19] MUNSON R A. The synthesis of copper disulfide [J]. Inorganic Chemistry, 1966, 5(7): 1296–1297. doi: 10.1021/ic50041a055 [20] BAYLISS P. Crystal chemistry and crystallography of some minerals within the pyrite group [J]. American Mineralogist, 1989, 74(9/10): 1168–1176. [21] UEDA H, NOHARA M, KITAZAWA K, et al. Copper pyrites CuS2 and CuSe2 as anion conductors [J]. Physical Review B, 2002, 65(15): 155104. doi: 10.1103/PhysRevB.65.155104 [22] KAKIHANA M, MATSUDA T D, HIGASHINAKA R, et al. Superconducting and fermi surface properties of pyrite-type compounds CuS2 and CuSe2 [J]. Journal of the Physical Society of Japan, 2019, 88(1): 014702. doi: 10.7566/JPSJ.88.014702 [23] KING H E, PREWITT C T. Structure and symmetry of CuS2 (pyrite structure) [J]. American Mineralogist, 1979, 64(11/12): 1265–1271. [24] MOSSELMANS J F W, PATTRICK R A D, VAN DER LAAN G, et al. X-ray absorption near-edge spectra of transition metal disulfides FeS2 (pyrite and marcasite), CoS2, NiS2 and CuS2, and their isomorphs FeAsS and CoAsS [J]. Physics and Chemistry of Minerals, 1995, 22(5): 311–317. doi: 10.1007/BF00202771 [25] FOLMER J C W, JELLINEK F, CALIS G H M. The electronic structure of pyrites, particularly CuS2 and Fe1- xCuxSe2: an XPS and Mössbauer study [J]. Journal of Solid State Chemistry, 1988, 72(1): 137–144. doi: 10.1016/0022-4596(88)90017-5 [26] TOSSELL J A, VAUGHAN D J, BURDETT J K. Pyrite, marcasite, and arsenopyrite type minerals: crystal chemical and structural principles [J]. Physics and Chemistry of Minerals, 1981, 7(4): 177–184. doi: 10.1007/BF00307263 [27] HÜPEN H, WILL G, HÖFFNER C, et al. X-ray diffraction of CuS2 under high pressure [J]. Materials Science Forum, 1991, 79/80/81/82: 697–702. doi: 10.4028/www.scientific.net/MSF.79-82.697 [28] NIWA K, TERABE T, SUZUKI K, et al. High-pressure stability and ambient metastability of marcasite-type rhodium pernitride [J]. Journal of Applied Physics, 2016, 119(6): 065901. doi: 10.1063/1.4941436 [29] TSE J S, KLUG D D, UEHARA K, et al. Elastic properties of potential superhard phases of RuO2 [J]. Physical Review B, 2000, 61(15): 10029–10034. doi: 10.1103/PhysRevB.61.10029 [30] MAO H K, XU J, BELL P M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions [J]. Journal of Geophysical Research, 1986, 91(B5): 4673–4676. doi: 10.1029/JB091iB05p04673 [31] DATCHI F, LETOULLEC R, LOUBEYRE P. Improved calibration of the SrB4O7: Sm2+ optical pressure gauge: advantages at very high pressures and high temperatures [J]. Journal of Applied Physics, 1997, 81(8): 3333–3339. doi: 10.1063/1.365025 [32] HAMMERSLEY A P, SVENSSON S O, HANFLAND M, et al. Two-dimensional detector software: from real detector to idealised image or two-theta scan [J]. High Pressure Research, 1996, 14(4/5/6): 235–248. doi: 10.1080/08957959608201408 [33] GONZALEZ-PLATAS J, ALVARO M, NESTOLA F, et al. EosFit7-GUI: a new graphical user interface for equation of state calculations, analyses and teaching [J]. Journal of Applied Crystallography, 2016, 49(4): 1377–1382. doi: 10.1107/S1600576716008050 [34] TOBY B H, VON DREELE R B. GSAS-II: the genesis of a modern open-source all purpose crystallography software package [J]. Journal of Applied Crystallography, 2013, 46(2): 544–549. doi: 10.1107/S0021889813003531 [35] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple [J]. Physical Review Letters, 1996, 77(18): 3865–3868. doi: 10.1103/PhysRevLett.77.3865 [36] ECKERT B, SCHUMACHER R, JODL H J, et al. Pressure and photo-induced phase transitions in sulphur investigated by Raman spectroscopy [J]. High Pressure Research, 2000, 17(2): 113–146. doi: 10.1080/08957950008200934 [37] PEIRIS S M, SWEENEY J S, CAMPBELL A J, et al. Pressure-induced amorphization of covellite, CuS [J]. The Journal of Chemical Physics, 1996, 104(1): 11–16. doi: 10.1063/1.470870 [38] ANASTASSAKIS E, PERRY C H. Light scattering and ir measurements in XS2 pryite-type compounds [J]. The Journal of Chemical Physics, 1976, 64(9): 3604–3609. doi: 10.1063/1.432711 [39] VOGT H, CHATTOPADHYAY T, STOLZ H J. Complete first-order Raman spectra of the pyrite structure compounds FeS2, MnS2 and SiP2 [J]. Journal of Physics and Chemistry of Solids, 1983, 44(9): 869–873. doi: 10.1016/0022-3697(83)90124-5 [40] SOURISSEAU C, CAVAGNAT R, FOUASSIER M. The vibrational properties and valence force fields of FeS2, RuS2 pyrites and FeS2 marcasite [J]. Journal of Physics and Chemistry of Solids, 1991, 52(3): 537–544. doi: 10.1016/0022-3697(91)90188-6 [41] THOMPSON E C, CHIDESTER B A, FISCHER R A, et al. Equation of state of pyrite to 80 GPa and 2 400 K [J]. American Mineralogist, 2016, 101(5): 1046–1051. doi: 10.2138/am-2016-5527 [42] BRAZHKIN V V, DZHAVADOV L N, EL'KIN F S. Study of the compressibility of FeSi, MnSi, and CoS2 transition-metal compounds at high pressures [J]. JETP Letters, 2016, 104(2): 99–104. doi: 10.1134/S0021364016140083 [43] YUY G, ROSS N L. Prediction of high-pressure polymorphism in NiS2 at megabar pressures [J]. Journal of Physics: Condensed Matter, 2010, 22(23): 235401. doi: 10.1088/0953-8984/22/23/235401 -






 下载:
下载: