Influence of Oxygen Concentration on Carbon Nanospheres Prepared by Gaseous Detonation
-
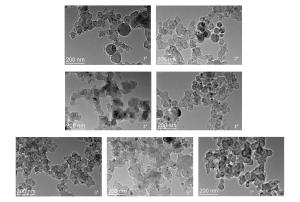
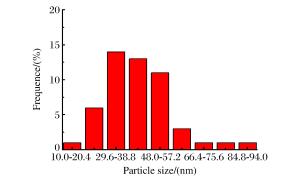
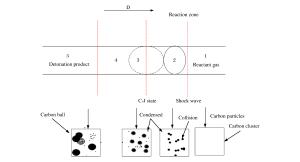
摘要: 以氧气和苯为反应物,采用气相爆轰法制备纳米碳球是一种新兴的碳纳米材料的制备方法,通过X射线衍射(XRD)进行物相分析,并通过透射电镜(TEM)进行形貌分析,观察了产物的晶粒大小。结果表明,纳米碳球的尺寸在30~50 nm之间,随着氧气浓度的增加,产物晶粒尺寸变小,分散性也变好,团聚程度降低。同时发现,反应物的初始浓度对气相爆轰合成纳米材料有重要影响。此外,还对气相爆轰合成纳米碳球的形成机理进行了讨论。Abstract: Preparing carbon nanospheres by gaseous detonation using oxygen-benzene mixture as the reactants is a new method. The crystal composition, grain size and phase structure were characterized by X-ray diffraction (XRD) and transmission electron microscopy (TEM). The results indicate that the dimension distribution of the carbon nanospheres ranges from 30 to 50 nm. The grain size decreases as the oxygen content increases, but the grain dispersivity is improved and the agglomerate is reduced. At the same time, the initial concentration of the reactants has significant influence on the synthesis of nanomaterial prepared by gaseous detonation. Furthermore, the growth mechanism of carbon nanospheres by gaseous detonation was discussed.
-
Key words:
- gaseous detonation /
- carbon nanospheres /
- grain size
-
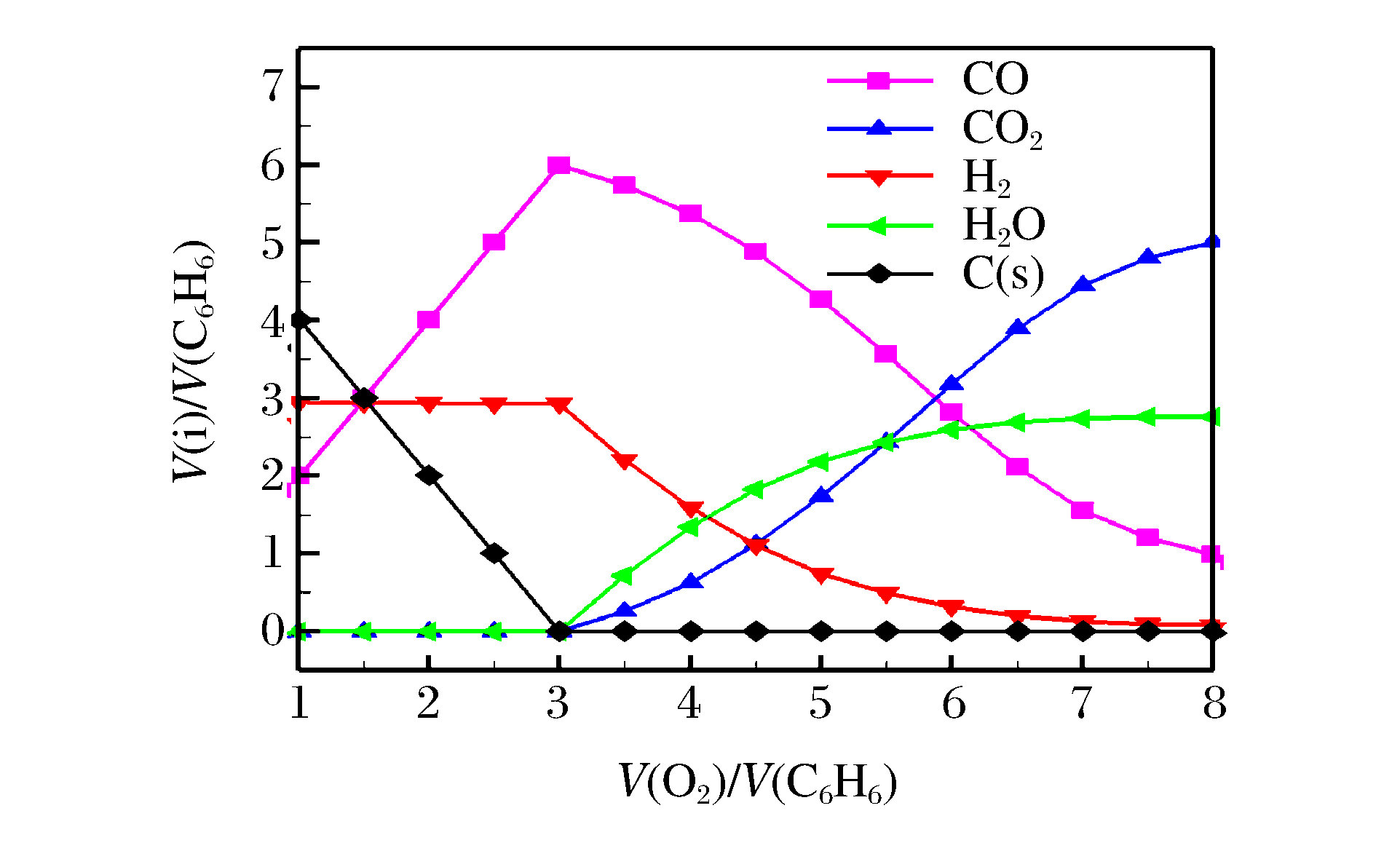
表 1 爆炸产物主要成分分布
Table 1. Distribution of main products from explosion
V(O2)/V(C6H6) OB Main ingredients of explosion products 1.0-3.0 - CO(g), H2(g), C(s) 3.0-7.5 - CO2(g), CO(g), H2O(g), H2(g) 7.5-8.0 + CO2(g), H2O(g), O2(g) 表 2 实验时的合成条件和反应参数
Table 2. Synthesis condition for samples and reaction parameters
No. Oxygen tension
/(MPa)Volume of
benzene/(mL)Benzene vapor partial
pressure/(MPa)n(O2/C6H6) 1 0.048 4.0 0.018 83 2.55 2 0.045 4.0 0.018 83 2.39 3 0.040 4.0 0.018 83 2.12 4 0.030 4.0 0.018 83 1.59 5 0.030 3.4 0.016 00 1.87 6 0.028 3.4 0.016 00 1.75 7 0.027 3.4 0.016 00 1.69 8 0.020 3.4 0.016 00 1.25 表 3 不同工况下的晶粒尺寸
Table 3. Crystalline size under different conditions
No. 2θ/(°) n(O2/C6H6) Crystalline
size/(nm)1 26.4 2.55 38 2 26.4 2.39 42 3 26.4 2.12 43 4 26.4 1.59 45 5 26.3 1.87 33 6 26.3 1.75 37 7 26.3 1.69 39 8 1.25 Incomplete detonation -
[1] CAI P J, FENG L.Synthesis of hollow carbon spheres by one convenient method[J].Mater Chem Phys, 2008, 108(1):1-3. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4837d13816cf59838713ff7ac9c8494a [2] 沈曾发.新型碳材料[M].北京:化学工业出版社, 2003.SHEN Z F.New carbon materials[M].Beijing:Chemical Industry Press, 2003. [3] LIU Y C, QIU X P, HUANG Y Q, et al.Mesocarbon microbeads supported Pt-Ru catalystsfor electrochemical oxidation of methanol [J].J Power Sources, 2002, 111(1):160-164. doi: 10.1016/S0378-7753(02)00298-7 [4] ZHANG C G, LI J J, LIU E, et al.Synthesis of hollow carbon nano-onions and their use for electrochemical hehydrogen storage [J].Carbon, 2012, 50(10):3513-3521. doi: 10.1016/j.carbon.2012.03.019 [5] GENG B Y, MA J Z, DU Q B, et al.Synthesis of hollow carbon nanospheres through a ZnSe nanoparticle template route[J].Mater Sci Eng A, 2007, 466(1):96-100. http://www.sciencedirect.com/science/article/pii/S0921509307006879 [6] TANG K, WHITE R J, MU X K, et al.Hollow carbon nanospheres with a high rate capability for lithium-based batteries [J].Chem Sus Chem, 2012, 5(2):400-403. doi: 10.1002/cssc.201100609 [7] TANG K, FU L J, WHITE R J, et al.Hollow carbon nanospheres with superior rate capability for sodium-based batteries [J].Adv Energ Mater, 2012, 2(7):873-877. doi: 10.1002/aenm.201100691 [8] HAN F D, YAN B, BAI Y J.Preparation of carbon nano-onions and their application as anode materials for rechargeable lithium-ion batteries [J].J Phys Chem C, 2011, 115(18):8923-8927. doi: 10.1021/jp2007599 [9] 季晶晶, 王红强, 张经济, 等.水热法制备Sn/C球复合材料及其电化学性能[J].粉末冶金材料科学与工程, 2013, 18(4):599-603. doi: 10.3969/j.issn.1673-0224.2013.04.022JI J J, WANG H Q, ZHANG J J, et al.Eletrochemical performance of Sn/C ball composite materials prepared by hydrothermal method[J].Powder Metallurgy Materials Science and Engineering, 2013, 18(4):599-603. doi: 10.3969/j.issn.1673-0224.2013.04.022 [10] PECH D, BRUNET M, DUROU H, et al.Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon [J].Nature Nanotech, 2010, 5(9):651-654. doi: 10.1038/nnano.2010.162 [11] 徐惠娟, 熊翔, 易茂中, 等.薄毡叠层炭/炭复合材料的高温导热性能[J].中南大学学报(自然科学版), 2008, 39(3):500-505. http://www.cnki.com.cn/Article/CJFDTOTAL-ZNGD200803016.htmXU H J, XIONG X, YI M Z, et al.Thermal conductivity properties of carbon/carbon composites with thin felt laminate at high temperature[J].Journal of Central South University (Natural Science Edition), 2008, 39(3):500-505. http://www.cnki.com.cn/Article/CJFDTOTAL-ZNGD200803016.htm [12] UGARTE D.Morphology and structure of graphitic soot particles generated in arc-discharge C60 production[J].Chem Phys Lett, 1992, 198(6):596-602. doi: 10.1016/0009-2614(92)85035-9 [13] WANG Q, CAO F Y, CHEN Q W, et al.Preparation of carbon micro-spheres by hydrothermal treatment of methylcellulose sol[J].Mater Lett, 2005, 59(28):3738-3741. doi: 10.1016/j.matlet.2005.06.046 [14] 李新海, 何方勇, 郭华军, 等.F-掺杂LiMn2O4的合成及电化学性能[J].中国有色金属学报, 2008, 18(1):55-58. http://d.old.wanfangdata.com.cn/Periodical/zgysjsxb200801010LI X H, HE F Y, GUO H J, et al.Synthesis and electrochemical performance of LiMn2O4 doped with F[J].The Chinese Journal of Nonferrous Metals, 2008, 18(1):55-58. http://d.old.wanfangdata.com.cn/Periodical/zgysjsxb200801010 [15] YOON S B, SOHN K, KIM J Y, et al.Fabrication of carbon capsules with hollow macroporous core/mesoporous shell structures[J].Adv Mater, 2002, 14(1):19-21. doi: 10.1002/(ISSN)1521-4095 [16] SHARON M, YASE K, MUKHOPADHYAY K, et al.Spongy carbon nanobeads-a new material[J].Carbon, 1998, 36(5):507-511. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ025288623/ [17] 文富国, 尹彩流, 黄启忠, 等.碳化钙-氯仿体系制备碳纳米球及其结构表征[J].广西民族大学学报(自然科学版), 2009, 15(1):67-69. doi: 10.3969/j.issn.1673-8462.2009.01.020WEN F G, YIN C L, HUANG Q Z, et al.Calcium carbide-chloroform system preparation of carbon nanotubes and their structural characterization[J].Journal of Guangxi University for Nationalities(Natural Science Edition), 2009, 15(1):67-69. doi: 10.3969/j.issn.1673-8462.2009.01.020 [18] SOGABE T, OKADA O, KURODA K, et al.Improvement in properties and air oxidation resistance of carbon materials by boron oxide impregnation[J].Carbon, 1997, 35(1):67-72. doi: 10.1016/S0008-6223(96)00128-5 [19] KROTO H W, MCKAY K.The formation of quasi-icosahedral spiral shell carbon particles[J].Nature, 1988, 331(228):328-331. doi: 10.1038/331328a0 -







 下载:
下载: